Figure 6.
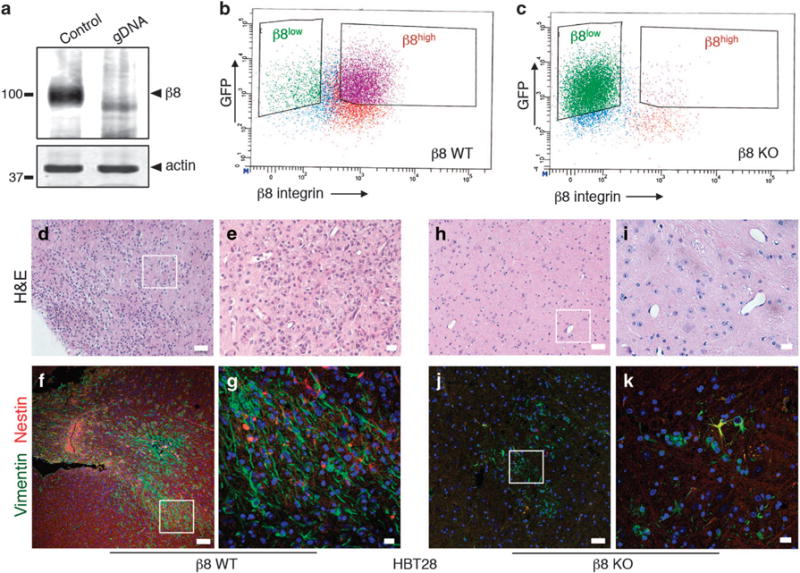
Genetically targeting β8 integrin in low-passage GBM cells leads to defective tumor initiation in vivo. (a) Immunoblot analysis showing absence of β8 integrin protein expression in β8KO cells generated via Crispr-Cas9 gene editing strategies. (b, c) FACS plot from β8WT (b) and β8KO (c) GBM cells, revealing loss of β8 integrin protein expression on the GBM cell surface. (d, e) Images of H&E-stained tumor sections from mice injected with β8WT GBM cells, revealing larger and more invasive tumors derived from β8WT cells. (f, g) Immunofluorescence analysis of xenograft tumors derived from β8WT GBM cells. The anti-vimentin antibody (green) is specific for human cells and anti-nestin (red) labels neural stem cells. (h, i) Brain sections from mice injected with β8KO GBM cells were stained with H&E, revealing small and minimally invasive tumors. (j, k) Immunofluorescence analysis with anti-vimentin and anti-nestin antibodies of mouse brains injected with β8KO GBM cells. Note that there are fewer human cells in β8KO-derived tumors as revealed by anti-vimentin immunofluorescence staining. All scale bars are 10 μm.
