Sessile plants are equipped with sophisticated mechanisms to integrate complex environmental signals into the endogenous program for adaptation to surrounding environment. How plants coordinately respond to environmental and endogenous signals is a central question for plant biologists. Plants respond to light signals through an exquisite array of photoreceptors that perceive and respond to a range of wavelengths of ambient light. These include UVR8 (for UV-B light), Cryptochromes and phototropins (for UV-A/blue light), and phytochromes (for red/far-red light). These photoreceptors not only help plants to track seasonal changes by sensing the wavelength, intensity, duration and the direction of incoming light signal, but also help to detect neighbors and shade conditions to modulate growth and development accordingly. At the seedling stage, a primary function of these receptors is to oppositely control cell expansion in two different organs, i.e, suppression of hypocotyl cells and expansion of cotyledon cells. A number of phytohormones, including auxin, also control cell expansion, although not oppositely in hypocotyls and cotyledons. Auxin is a small indolic compound with a tremendous regulatory role in plant growth and development. It controls various responses in part by inducing rapid changes in gene expression. Two families of transcription factors play central, albeit antagonistic roles in auxin-induced gene expression: AUX/IAA proteins and ARF proteins. AUX/IAA proteins can form heterodimers with ARF proteins and negatively regulate the transcriptional activity of the ARF proteins through their potent repressor domains (Mockaitis and Estelle, 2008). To activate transcription, auxin is perceived by the TIR1/AFB family of F-box proteins and AUX/IAA co-receptors. This results in formation of the SCF (SKP1, Cullin and F-box proteins) complex that functions as an E3 ligase. The SCFTIR1/AFB complex tags AUX/IAA proteins with polyubiquitination, which is then recognized by the 26S proteasome for subsequent degradation (Mockaitis and Estelle, 2008). Therefore, auxin directly participates in the destruction of negative regulators, AUX/IAA proteins, allowing ARF proteins to mediate rapid auxin-induced gene expression. An attractive hypothesis is whether photoreceptor signaling pathways control cell expansion in these organs directly or indirectly by modifying auxin levels and/or signaling. Several pieces of early evidence suggest that one mode of light signaling may differentially control cell expansion in these organs by modifying auxin signaling (Colon-Carmona et al., 2000; Tian et al., 2003). However, a direct evidence about the molecular mechanisms by which light signaling pathways modulate auxin signaling to optimize plant growth and development was unknown until recently.
Direct physical interactions between photoreceptors and AUX/IAA proteins
Two recent complementary discoveries highlight the direct molecular convergence of light and auxin signaling pathways to regulate plant growth and development. Xu et al used seedling de-etiolation as a model system to show that both CRY1 and phyB directly interact with multiple AUX/IAA proteins, and regulate their abundance in response to blue and red-light signals, respectively (Xu et al., 2018). They used a variety of methods to demonstrate direct physical interactions between CRY1-AUX/IAA (under blue light) and phyB-AUX/IAA proteins (under red light). They demonstrate that these interactions result in the stabilization of AUX/IAA proteins under blue and red light conditions. Moreover, they showed that the interactions between CRY1-AUX/IAA and phyB-AUX/IAA prevent interactions between TIR1-AUX/IAA proteins even in the presence of auxin, suggesting that the photoreceptors outcompete the auxin receptors, TIR1/AFB for binding to AUX/IAA proteins. The stable AUX/IAA proteins then interact with ARF transcription factors and inhibit ARF-induced gene expression to prevent cell elongation at the seedling stage, resulting in shorter hypocotyls under blue and red-light conditions compared to darkness. Consistent with these data, the gene expression profiling shows antagonistic regulation of gene expression by the auxin receptors and photoreceptors in response to auxin and light signals, respectively. Finally, cry1 and phyB mutants display hyposensitive phenotypes in response to blue and red light-mediated inhibition of auxin signaling pathways. These data also provide a molecular explanation of a previous study that showed the biological function of the N-terminal domain of CRY1 in mediating blue-light induced inhibition of hypocotyl elongation (He et al., 2015). Thus, both the N- and C-terminal domains of CRY1 promote seedling de-etiolation, but they do so by two distinct mechanisms. N-terminal domain of CRY1 stabilizes AUX/IAA proteins to inhibit cell elongation while the C-terminal domain interact and inhibit the COP1-SPA1 complex to stabilize HY5 (Yang et al., 2000), which in turn inhibits hypocotyl elongation in response to light. Overall, these data highlight the direct convergence of light and auxin signaling pathways to regulate seedling de-etiolation.
Yang et al used a well-known physiological phenomenon known as the shade avoidance response (SAR) as the basis of their study (Yang et al., 2018). In contrast to regular sunlight that has high red:far-red (R:FR) ratio (~1.2–1.5), canopy shade and/or high-density planting conditions result in low to very low R:FR ratio (<0.7 to <0.05) (Roig-Villanova and Martínez-García, 2016; Yang et al., 2018). The altered R:FR ratio is perceived by phytochromes (mainly phyB and phyA). Low R:FR induces a conformational change of phyB from a biologically active Pfr to an inactive Pr form, while phyA is slightly stabilized (Yang et al., 2018). Under these conditions, plants elongate their hypocotyls/stems and petioles at the expense of leaf size and root elongation, resulting in decreased biomass and early flowering. Under deep canopy shade conditions (R:FR ratio ~ <0.05), phyB is still in the inactive Pr form. However, phyA is strongly stabilized due to a reduced degradation rate and is converted to an active Pfr form, which inhibits hypocotyl/stem and petiole elongation (Roig-Villanova and Martínez-García, 2016; Yang et al., 2018). phyB is known to regulate auxin biosynthesis as well as signaling, albeit indirectly through regulation of PIF4/PIF5/PIF7 (Wit et al., 2016). By contrast, the new discovery shows that phyA does not play a role in regulating auxin biosynthesis (Yang et al., 2018); rather, the active Pfr form of phyA directly interacts with AUX/IAA proteins and stabilizes them under deep canopy shade conditions. Similar to CRY1 and phyB, phyA also competes with TIR1/AFBs to bind to AUX/IAA proteins. As discussed above, these stable AUX/IAA proteins then interact with ARF transcription factors to inhibit auxin-induced gene expression and hypocotyl/stem elongation. Consistently, phyA mutant also displays hypersensitive phenotype to auxin-induced hypocotyl elongation presumably due to a reduced level of AUX/IAA proteins. The above studies highlight how different photoreceptor families provide adaptive advantages to plants under different wavelengths of light environment.
Other unresolved questions in light and auxin signaling pathways
One of the intriguing findings in the above studies is that both CRY1 and phyA/phyB directly interact with AUX/IAA proteins and stabilize them without sequestering. The stabilized AUX/IAA proteins then interact with ARF and inhibit auxin-regulated gene expression resulting in inhibition of cell elongation. By contrast, phyB has been shown to directly interact with PHYTOCHROME INTERACTING FACTORs (PIFs) and sequester them in response to red light (Park et al., 2012). It is unclear why phyB sequesters PIFs while releases AUX/IAA proteins after interaction. One possibility is that phyB has a much higher affinity for PIFs than AUX/IAA proteins and the differences in affinities might explain sequestration vs release after interaction. Alternatively, both CRY1 and phyA/phyB might post-translationally modify these proteins in a way that they are released after interactions. Photoreceptors might induce phosphorylation of AUX/IAA proteins as has been shown previously for phyA (Colon-Carmona et al., 2000), and the phosphorylated form is then released for interaction with ARF proteins. Furthermore, both studies used seedling as a model system. Do these regulatory mechanisms also function similarly in adult plants? Canopy shade and high-density planting not only affect R:FR ratio, but also induce other responses including touch responses. Further studies are necessary to understand whether similar pathways function under these conditions.
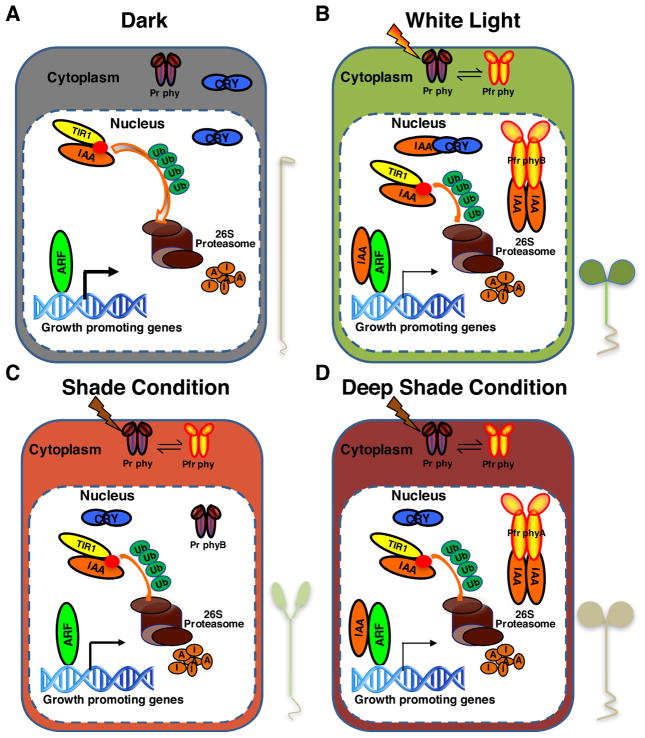
Figure 1.
A proposed model for direct convergence of light and auxin signaling pathways.
(A) In the dark, phytochromes are in an inactive Pr form located in the cytoplasm, while CRY1 is located both in the cytoplasm and nucleus. TIR1/AFBs form an SCF complex to induce degradation of AUX/IAA proteins. ARF transcription factors bind to DNA and promote auxin-induced gene expression and hypocotyl elongation. (B) Under white light with high R:FR ratio, phyA is degraded while phyB is converted to the Pfr form, which migrates into nucleus. Both phyB Pfr form and CRY1 interact with AUX/IAA proteins and stabilize them. Stable AUX/IAAs inhibit auxin-induced gene expression resulting in short hypocotyls. (C) Under shade and high-density planting conditions, low R:FR ratio results in inactivation of phyB. TIR1/AFBs induce degradation of AUX/IAA proteins, resulting in auxin-induced gene expression and elongation of hypocotyls. (D) Under deep canopy shade conditions, phyA is stabilized and is converted to an active Pfr form, which interacts with AUX/IAA proteins and stabilize them. Stable AUX/IAAs inhibit ARF activity and suppression of auxin-induced cell elongation. Thus, seedlings display short hypocotyl under deep canopy shade conditions. Thickness of the arrows indicates transcription activity of the ARF proteins.
 indicates auxin molecule.
indicates auxin molecule.
Acknowledgments
Funding
This work was supported by grants from National Institute of Health (NIH) (1R01 GM-114297) and National Science Foundation (MCB-1543813) to E. H.
We thank members of the Huq laboratory for critical reading of the manuscript.
Footnotes
The author does not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Colon-Carmona A, Chen DL, Yeh KC, Abel S. IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 2000;124:1728–1738. doi: 10.1104/pp.124.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SB, Wang WX, Zhang JY, Xu F, Lian HL, Li L, Yang HQ. The CNT1 Domain of <em>Arabidopsis</em> CRY1 Alone Is Sufficient to Mediate Blue Light Inhibition of Hypocotyl Elongation. Molecular Plant. 2015;8:822–825. doi: 10.1016/j.molp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. Auxin Receptors and Plant Development: A New Signaling Paradigm. Annu Rev Cell Dev Biol. 2008;24:55–80. doi: 10.1146/annurev.cellbio.23.090506.123214. [DOI] [PubMed] [Google Scholar]
- Park E, Park J, Kim J, Nagatani A, Lagarias JC, Choi G. Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J. 2012;72:537–546. doi: 10.1111/j.1365-313X.2012.05114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I, Martínez-García JF. Plant Responses to Vegetation Proximity: A Whole Life Avoiding Shade. Frontiers in Plant Science. 2016;7:236. doi: 10.3389/fpls.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Nagpal P, Reed JW. Regulation of Arabidopsis SHY2/IAA3 protein turnover. Plant J. 2003;36:643–651. doi: 10.1046/j.1365-313x.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- Wit Md, Galvão VC, Fankhauser C. Light–Mediated Hormonal Regulation of Plant Growth and Development. Ann Rev Plant Biol. 2016;67:513–537. doi: 10.1146/annurev-arplant-043015-112252. [DOI] [PubMed] [Google Scholar]
- Xu F, He S, Zhang J, Mao Z, Wang W, Li T, Hua J, Du S, Xu P, Li L, et al. Photoactivated CRY1 and phyB Interact Directly with AUX/IAA Proteins to Inhibit Auxin Signaling in Arabidopsis. Molecular Plant. 2018 doi: 10.1016/j.molp.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Yang C, Xie F, Jiang Y, Li Z, Huang X, Li L. Phytochrome A Negatively Regulates the Shade Avoidance Response by Increasing Auxin/Indole Acidic Acid Protein Stability. Developmental Cell. 2018;44:1–13. doi: 10.1016/j.devcel.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR. The C Termini of Arabidopsis Cryptochromes Mediate a Constitutive Light Response. Cell. 2000;103:815–827. doi: 10.1016/s0092-8674(00)00184-7. [DOI] [PubMed] [Google Scholar]