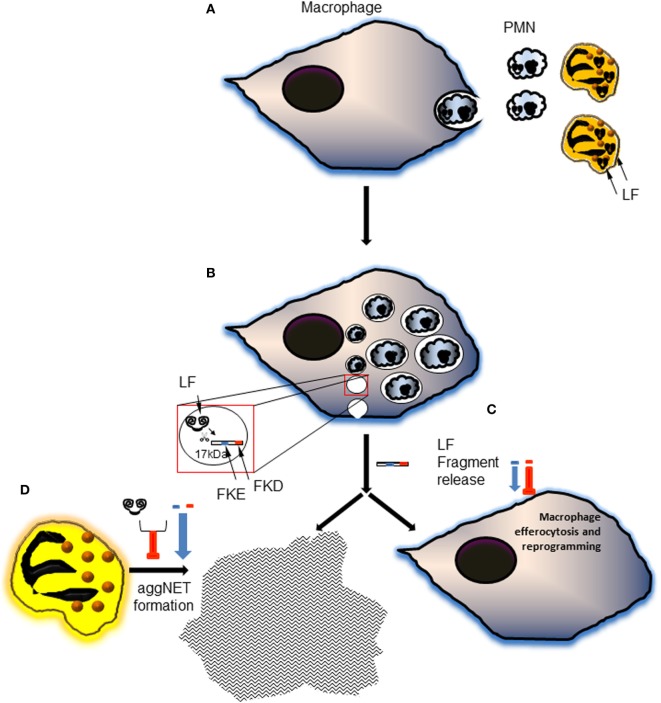
Figure 7.
A schematic illustration of the proposed model of generation and action of lactoferrin (Lf) fragments and peptides during the resolution of inflammation. (A) During inflammation and its resolution, neutrophils undergo apoptosis and get engulfed by macrophages. This process is accompanied by neutrophil elastase-mediated cleavage of Lf and generation of the 17-kDa fragment. (B) The engulfed polymorphonuclear cell (PMN) corpses traffic to the phagolysosome where most of their cellular components are hydrolyzed. Lf fragments are accumulating in resolution phase macrophages during the inflammatory and resolving phases of the response. 15- and 17-kDa fragments were produced during the inflammatory phase and the resolution of inflammation, respectively. The 17-kDa Lf fragment is released and possibly undergoes further processing to shorter peptides containing the tripeptides FKE and FKD. (C) Both peptides enhance macrophage conversion to the CD11blow phenotype, whereas FKE peptides enhance macrophage efferocytosis in vivo. FKE peptides also promote macrophage reprogramming in vivo culminating in a reduced secretion of tumor necrosis factor α (TNFα) and interleukin (IL)-6, and an increased secretion of IL-10 upon exposure to LPS. (D) Lf peptides also promote the generation of pro-resolving aggregated neutrophil extracellular traps (aggNETs) in monosodium urate (MSU)-activated human neutrophils, while Lf fails to do so. Intriguingly, when combined with full-length Lf, Lf peptides enhance its inhibition of aggNETs formation following MSU exposure. Altogether, these findings suggest that Lf undergoes specific proteolytic processing to yield functional fragments that are released and “recycled”. These fragments contain bioactive peptides with anti-inflammatory and pro-resolving properties that orchestrate the resolution of neutrophil-driven inflammation.