Fig. 1.
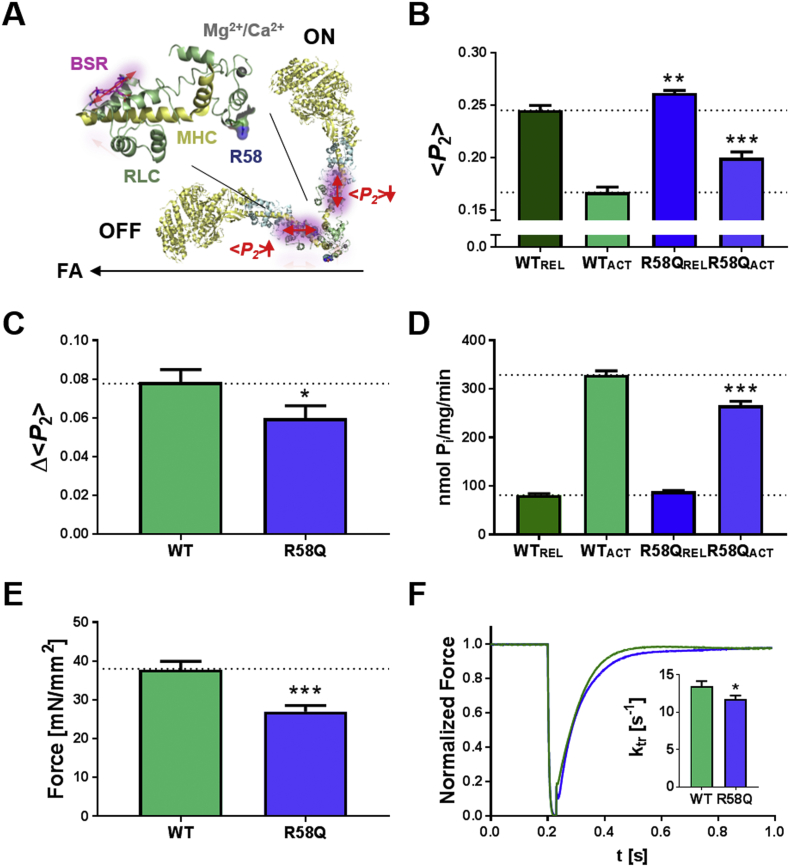
R58Q stabilizes the OFF conformation of the myosin heads. (A) Left: Structure of the RLC-region of myosin with RLC and myosin heavy chain (MHC) shown in green and yellow, respectively. Arginine 58 is shown in van-der-Waals representation, and the BSR probe attached to the E-helix and putative Ca2+/Mg2+ binding site are shown in purple and grey, respectively. Right: OFF and ON orientations of the myosin heads with respect to the filament axis (FA; black arrow), reported by parallel (high value of the order parameter <P2>) and perpendicular (low <P2>) orientations of the E-helix probe, respectively. (B) <P2 > measured from WT- (green) and R58Q-BSR-cRLC-E (blue) exchanged ventricular trabeculae in relaxing conditions (REL, pCa 9) and full calcium activation (ACT, pCa 4.3) at ~1.9 μm sarcomere length. (C) Decrease in <P2 > (Δ < P2>) on calcium activation for WT- and R58Q-BSR-cRLC-E exchanged trabeculae. (D) ATPase activity of cardiac myofibrils in relaxing conditions and full calcium activation exchanged with either WT- (green) or R58Q-RLC (blue). (E) Maximum isometric force and (F) rate of force re-development of WT- and R58Q-BSR-cRLC-E exchanged ventricular trabeculae. Means ± SEM (n = 4–16). Statistical significance of difference between WT and R58Q groups was assessed with a two-tailed unpaired Student's t-test: *p < .05, **p < .01, ***p < .001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)