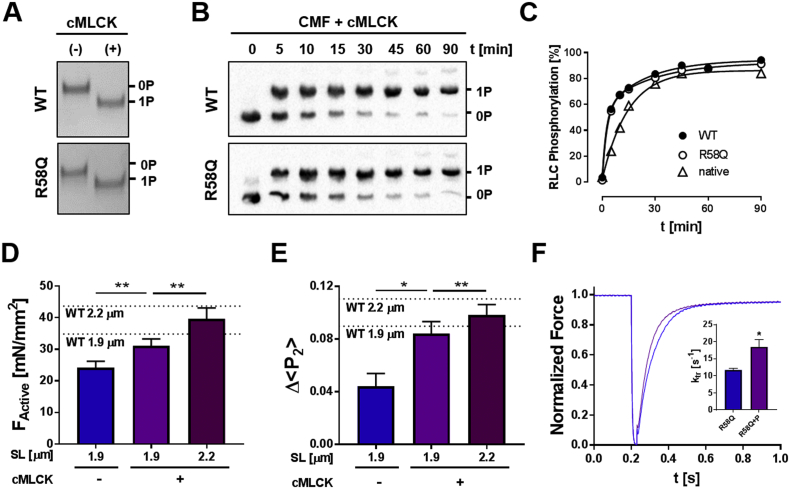
Fig. 4.
RLC phosphorylation restores myofilament function in the presence of R58Q-RLC. (A) In vitro kinase assay of WT- and R58Q-RLC incubated without (−) and with cMLCK (+) and analyzed by urea-glycerol PAGE. (B) In situ phosphorylation of WT- and R58Q-RLC exchanged into cardiac myofibrils (CMF) analyzed by Phostag™-SDS-PAGE and Western blot against RLC. (C) Time-course of phosphorylation of native, and WT- and R58Q-RLC exchanged CMFs. (D) Active isometric force (FActive) and (E) Δ < P2 > of R58Q-BSR-cRLC-E exchanged trabeculae before (−) and after RLC phosphorylation (+) to ~0.3 mol Pi/mol RLC (Fig. S6) at short sarcomere length (~1.9 μm), and after RLC phosphorylation at long sarcomere length (~2.2 μm). The values obtained for WT-BSR-cRLC-E at each sarcomere length are indicated by dotted lines and labelled accordingly. (F) Rate of force re–development of R58Q-BSR-cRLC-E exchanged trabeculae before (blue) and after RLC phosphorylation (purple). Means ± SEM (n = 4–7). Statistical significance of differences of values were assessed with a two-tailed paired Student's t-test: *p < .05, **p < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)