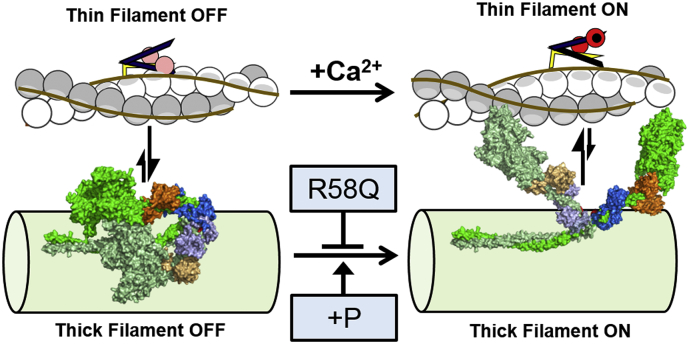
Fig. 5.
Model for the effect of R58Q on myofilament activation in the heart. Cardiac contraction is controlled by regulatory structural transitions in both actin-containing thin and myosin-containing thick filaments. During diastole (left) both thin and thick filament are in the OFF state. Tropomyosin (brown lines) blocks myosin-binding sites on actin (white and grey circles), and myosin head domains (space filling representation, green) are sequestered on the surface of the thick filament backbone. During systole (right) calcium (black circle) binds to troponin C (red) and activates the thin filaments by removing inhibitory interactions of troponin I (yellow) and troponin T (purple) with actin, followed by tropomyosin moving azimuthally away from myosin-binding sites on actin. Calcium activation of the thin filament activates myosin heads through so far unknown mechanisms (indicated by vertical arrows), resulting in release of the heads from the surface of the thick filament to become available for actin binding and force-generation. The R58Q mutation in the myosin RLC (blue) reduces cardiac contractility by stabilizing the thick filament OFF state and preventing myosin heads from leaving the thick filament surface. RLC phosphorylation (+P) restores thick filament activation in the presence of the R58Q mutation by destabilizing the OFF state and promoting the myosin head ON state. Homology models for human β-cardiac myosin were created by the Spudich laboratory (http://spudlab.stanford.edu/homology-models/). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)