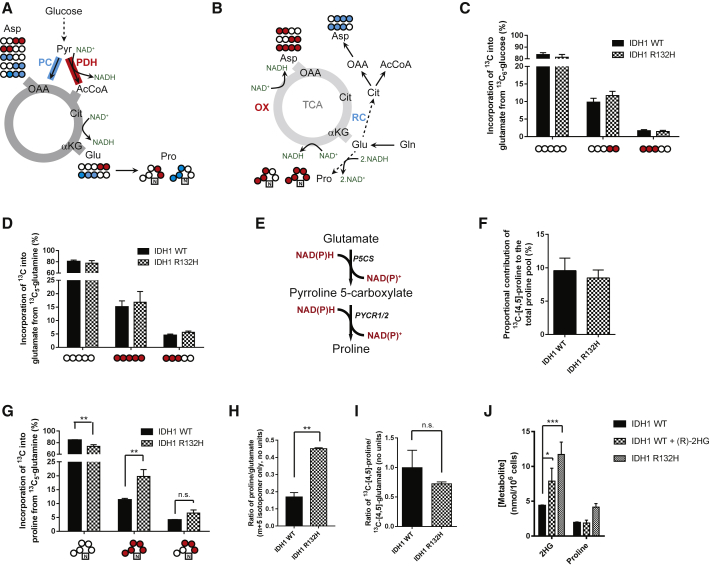
Figure 1.
Increased Proline Synthesis in IDH1 Mutant Cells
(A) Alternative entry points of pyruvate into the TCA cycle and the implications for 13C incorporation into metabolites from 13C6-glucose.
(B) Incorporation of 13C5-glutamine into the TCA cycle.
(C) Incorporation of glucose-derived pyruvate into glutamate shows no difference between WT and IDH1 R132H-expressing cells.
(D) 13C incorporation into glutamate from 13C5-glutamine also shows no significant differences between IDH1 mutant and WT cells.
(E) Diagram showing the synthesis of proline from glutamate, with 2 mol NAD(P)H oxidized per mole of glutamate to proline.
(F) The contribution of glucose carbons to the proline pool is unchanged in cells expressing mutant IDH1.
(G) Glutamine carbons that arise directly from glutamate (without a pass through the TCA cycle) are significantly increased as a proportion of the proline pool. Absolute values were calculated from 1H-NMR spectra.
(H and I) In (H), when the proline synthesized from glutamine is normalized to take into account relative changes in glutamate labeling (m + 5 proline/m + 5 glutamate; gas chromatography-mass spectrometry [GC-MS]), this significance is retained, while (I) 13C-[4,5]-proline arising from 13C-[4,5]-glucose remains unchanged (HSQC NMR spectra).
(J) IDH1 WT cells incubated with 10 mM (R)-2HG elicits an intermediate 2HG intracellular concentration that does not increase proline synthesis (calculated from 1H NMR spectra).
All error bars represent mean ± SEM. Statistical tests used for both (G) and (I): 2-way ANOVA with post hoc test to identify individual significant changes. In (H) and (I), comparisons were performed using a Mann-Whitney test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant. αKG, α-ketoglutarate; AcCoA, acetyl coenzyme A; Asp, aspartate; Cit, citrate; Glu, glutamate; OAA, oxaloacetate; OX, oxidative TCA cycle; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; Pro, proline; RC, reductive carboxylation; TCA, tricarboxylic acid.