Abstract
Aim
To assess outcome of TAVI in high risk patients with severe symptomatic aortic stenosis.
Patients and methods
40 patients with symptomatic severe aortic stenosis and high risk underwent TAVI with implantation of either Sapien XT valve or Core Valve and followed for 6 months. Device success, cardiovascular mortality, myocardial infarction, stroke, life-threatening bleeding and vascular complications were defined according to Valve Academic Research Consortium definitions.
Results
The study included 40 patients, their mean age was 73.98 ± 8.40, procedural success was 97.5%. One patient need valve in valve due to moderately severe paravalvular leak. Total mortality was 7.5%, cardiovascular death occurred in 2.5% and non cardiovascular death occurred in 5%. Myocardial infarction occurred in one patient (2.5%), stroke occurred in 2 patients (5%), minor bleeding occurred in 6 patients (15%), major bleeding occurred in 3 patients (7.5%), minor vascular complications occurred in 4 patients (10%) while major vascular complications occurred in 3 patients (7.5%). Permanent pacemaker was inserted for 5 patients (12.5%), new onset AF occurred in 4 patients (10%). Re hospitalization was needed for 2 patients (5%) due to heart failure. After TAVI there were significant improvement in NYHA functional class (p < 0.001), mean LV ejection fraction and LV mass index (p < 0.001), mean aortic valve area, mean and peak pressure gradient (p < 0.001), severity of aortic and mitral regurgitation (p < 0.001). When comparing types of valves used, both were nearly comparable.
Conclusion
TAVI is a safe and effective procedure in selected high-risk patients with severe symptomatic aortic stenosis without significant difference between used valves.
Keywords: Transcatheter aortic valve implantation, Aortic stenosis, Outcome
1. Introduction
Prolonged average life expectancy has resulted in an aging population and subsequent aortic valve calcification. Calcified degenerative aortic valve stenosis is currently the most frequent native valve disease. Furthermore, it is most often seen in elderly patients with comorbidities.1
Once symptoms develop, in the form of angina, syncope or congestive heart failure, the prognosis in these patients deteriorates rapidly, with an estimated life expectancy of two to five years.2
Although current guidelines recommend surgical valve replacement as the therapy of choice in all symptomatic patients with severe AS, actually about one third of these patients are excluded or not considered for surgery because of inoperability or high surgical risk due to advanced age or multiple comorbidities.1
In patients without frank LV failure, the operative risk ranges from 2% to 5% in most centers and3 when operation is carried out in patients with critical AS, frank LV failure, depressed ejection fraction, low cardiac output or those with multiple comorbidities, the operative risk is higher and the mortality rate ranges from 8% to 20%.4
Since the initial implantation by Alain Cribier, TAVI has become an established therapy for patients with symptomatic severe aortic stenosis, who are deemed too high risk for surgical aortic valve replacement.5
PARTNER trial demonstrates that TAVI maintains a sustained superiority over medical treatment in inoperable patients with symptomatic severe aortic stenosis and equivalent outcomes between TAVI and SAVR in high-risk patients.5
1.1. Aim of the work
The study aimed to assess the outcome of transcatheter aortic valve implantation in high risk patients with severe symptomatic aortic stenosis.
1.2. Patients and methods
This study was a multicenter, observational, retrospective and prospective that was conducted between February 2013 and March 2016 in Cairo, Egypt for 40 patients presented by severe symptomatic AS and high surgical risk or inoperability criteria according to the EuroSCORE. Patients were considered for TAVI if their EuroSCORE II ranged from 15 to 20%.
Patients were scheduled to receive either Edwards SAPEIN XT valve or Core Valve revalving system valves.
Patients were followed for 6 months after TAVI procedure.
1.2.1. Inclusion criteria
-
•
Patient with severe symptomatic aortic stenosis who were considered at high risk for a surgical aortic valve replacement and their Euro SCORE is 15 to 20%.
1.2.2. Exclusion criteria
-
•
Patients with a recent neurological events, respiratory failure and liver cirrhosis.
-
•
Patients with heavily calcified, small tortuous ileo femoral arteries.
-
•
Patients with multivalvular heart diseases.
-
•
Patients with previous mitral valve surgery.
-
•
Patients with aortic annulus diameter ≤19 mm or >27 mm.
-
•
Patients with CHB and atrial fibrillation.
-
•
Patients with bicuspid aortic valve.
-
•
Patients with distance to the coronary artery ostium <11 mm.
-
•
Patients with significant aortic regurgitation (moderately severe AR).
1.2.3. Methods
For all potential candidates for TAVI, we performed a diagnostic screening in order to evaluate the eligibility to transcatheter procedure and to accurately assess vascular access to minimize the complications.
1.3. Pre operatively all patients were subjected to
1.3.1. History
Complete medical history with special emphasis on:
-
1-
The cardinal symptoms of acquired severe AS (dyspnea, angina, syncope, and ultimately heart failure symptoms).
-
2-Risk factors of atherosclerosis such as:
-
•Smoking.
-
•Hypertension
-
•Dyslipidemia.
-
•Diabetes mellitus.
-
•
-
3-
History of peripheral vascular disease.
-
4-
History of cerebrovascular accident.
-
5-
History of previous atrial fibrillation.
1.3.2. Full clinical examination
General examination: with attention to the vital signs (pulse, blood pressure, respiratory rate), all peripheral pulsations and signs of heart failure (lower limb edema, elevated jugular venous pressure, congested liver, dyspnea, orthopnea, ect.)
Local examination: with attention to heart sounds specially S3, ejection systolic murmur of aortic stenosis and diastolic murmur of aortic regurgitation over the base and systolic murmur of mitral regurgitation over the apex, sings of heart failure (galloping, bilateral basal crepitation, ect.)
1.3.3. Blood tests
-
•
Complete Blood Count.
-
•
Urea and creatinine, creatinine clearance ratio.
-
•
Coagulation profile.
-
•
Other routine laboratory investigations such as lipid profile (cholesterol, TG, LDL, HDL) and blood glucose level (HbA1c, fasting blood glucose two hour postprandial blood glucose) were done.
1.3.4. Electrocardiogram
We used the Fukuda 3 channel machine. Twelve leads ECG was done for all patients before TAVI, immediately after the procedure and during the hospital stay, at 30 days and at 6 month follow up visits.
The main ECG changes followed up were: ST-T changes especially new changes to determine presence and early detection of new ischemia, left ventricular hypertrophy, left atrial enlargement, AF and conduction abnormalities.
1.3.5. Echocardiography
1.3.5.1. 1-Transthoracic echocardiogram
We used the General Electric (GE) echocardiographic machine model 5500 with transducer of 3.5–5 MHz.
Before the procedure:
-
•
2-D echo used for assessment of cardiac chambers, volumes and by using the modified Simpson’s method the ejection fraction can be determined.
2-D echo also can be used for assessment of valve anatomy, aortic root, aortic annulus, valve calcification and to exclude those with bicuspid aortic valve and diagnosis of associated mitral valve disease.
M-mode echocardiography used for assessment of the LV dimensions and for calculation of ejection fraction. M – mode echocardiographic imaging also used for the evaluation of LV hypertrophy, LV mass, LV mass index and for measurement of aortic root dimensions.6
By M-mode the LV mass and LV mass indexed to body surface area estimated by LV cavity dimension and wall thickness at end-diastole.
LV mass and LV mass index calculator are available online.
LV Mass(g) = 0.8{1.04[([LVEDD + IVSd + PWd]3 − LVEDD3)]} + 0.6
LVEDD is the LV end diastolic diameter (mm).
IVSD is the interventricular septal thickness at the end diastole (mm).
PWT is the posterior wall thickness at the end diastole (mm).
- 1.04 is the specific gravity of the myocardium.7
-
•Doppler echocardiography provides measurement of the trans aortic jet velocity, which is the most useful measure for the disease severity. The mean and maximal trans aortic pressure gradient is calculated using the modified Bernoulli equation.
-
•
The Bernoulli equation:
Δ P = 4V2 While the maximum gradient equals Δ P max = 4V2 max.
P max = pressure gradient, v max = maximal velocity.8
The effective orifice area was calculated using the continuity equation;
As the AVA = aortic valve area, VTI = velocity time integral, CSA = cross sectional area, LVOT = left ventricular out flow tract.
-
•
The combination of pulsed, continuous wave and color flow Doppler echocardiography used in detecting and determining the location and severity of aortic regurgitation, detecting and determining the severity of mitral regurgitation and in estimating pulmonary artery pressure.
1.3.5.2. 2-Trans esophageal echocardiography
TEE was used during the procedure for measurement of the aortic root, the aortic annulus and to help in proper positioning of the prosthetic valve before deployment .TEE was used immediately after TAVI to assess the location and degree of aortic regurgitation and the patency of the coronary arteries and to rule out any complication.
1.3.6. Multislice computed tomography (CT)-scan
We used Siemens MSCT SOMATA definition machine 64 dual source (128 slice model). A multislice computed tomography was done before TAVI to assess the aortic root, ascending, thoracic and abdominal aorta, and also the iliac-femoral axis was evaluated. MSCT, also, provides greater appreciation of vessel diameter, tortuosity and calcification burden and measurement of the aortic annulus and measurement the distance to the coronary ostium.
1.3.7. Aortography
We used the Mark V ProVis INJECTOR SYSTEM. Aortography was performed at the beginning of the procedure and repeated during the procedure to determine the position of the valve and the plane of alignment of the aortic cusps, to assess the patency of the coronary arteries and to assess the presence or absence of AR and aortic dissection if occurred. Immediately after TAVI, aortography were performed to assess the location and degree of AR and to rule out complications.
1.3.8. Description of procedures
The procedure was performed under conscious sedation and/or local anesthesia at each groin prior to arterial puncture. Contralateral common femoral artery was punctured using Seldinger’s technique, followed by insertion of a 6 F Cordis 11 cm sheath. A 6 F pigtail catheter was advanced to level of aortic bifurcation and aortoiliac angiogram was performed.
The common femoral artery of ipsilateral side was punctured in the same way with placement of 8 F Cordis 11 cm sheath, which was exchanged for a Proglide vascular closure device that was predeployed in the arterial wall around puncture site.
Then a 10 F Cordis Sheath was inserted. A 6 F pigtail catheter was advanced from ipsilateral side to aortic root over a 0.035″ J tipped wire. The wire then was exchanged for a super stiff backup Meier wire to straighten ileofemoral vessels. The 10 F sheath was then exchanged for Edwards dilator followed by Edwards sheath in case of Edwards Sapien XT valve implantation, or for 14 F dilator followed by 18 F Cook sheath in case of CoreValve implantation. We used 16 F Edwards dilator for the 18 F Edwards sheath and 18 F Edwards dilator for 19 F Edwards sheath.
The contralateral pigtail catheter was then advanced to the aortic root and placed in the noncoronary sinus. A 6 F Amplatz left-1or 2 catheter was advanced over a wire from ipsilateral side to the aortic root. Crossing of the aortic valve was performed with AL-1 catheter assisted with a straight 0.035″ wire, which was exchanged for a 260 cm J tipped wire after crossing the aortic valve.
Then, AL-1 catheter was then exchanged for pigtail catheter, and simultaneous pressure measurements in LV and aortic root was performed. Then, a 260 cm pre-shaped Amplatz superstiff wire was placed in the LV and served as a railway for aortic valvuloplasty and valve implantation. Aortic valvuloplasty was performed under rapid right ventricular (RV) pacing at a rate of 180 bpm using Edwards balloon or Z-Med balloon with a diameter that is 3 mm less than that of intended prosthesis.
Prosthesis implantation was performed under rapid RV pacing at a rate of 180 bpm in case of Edwards Sapien XT valve or 110 bpm in case of CoreValve. The C arm position was adjusted during valve implantation to get a perpendicular view of the valve. Simultaneous LV and aortic root pressure measurements and control aortography were performed after valve implantation, and post dilatation was performed in exceptional cases, if needed to correct residual paravalvular leaks.
During prosthesis implantation rapid RV pacing aimed at nullifying effective cardiac output during balloon expandable valve implantation to avoid prosthesis dislocation. In case of self expandable CoreValve, the goal was to minimize the force of LV contraction during the initial phase of prosthesis implantation in order to facilitate prosthesis positioning.
Nullifying the cardiac output during CoreValve implantation is not necessary as prosthesis allows forward blood flow even before full deployment. Vascular access closure was performed by tightening on the Proglide sutures. The contralateral side was closed with another closure device. A compression bandage was applied over groin for 24 h.
Heparin at a dose of 100 IU/kg body weight is administered to yield an activated clotting time of 250–300 s throughout the procedure and after the procedure, the heparin was neutralized by protamine. Patients were pre-medicated with aspirin, clopidogrel.
1.3.9. Post-TAVI monitoring and management
After TAVI, patients remained in the cardiac care unit for at least 24 h and are closely monitored for 48–72 h with particular attention to hemodynamic balance, vascular access site, renal function, infections, bleeding and other complications. A transthoracic echocardiography, twelve-lead electrocardiography, chest X-ray was performed during the first 24 h after TAVI and according to clinical need.
Blood tests were carried out every 8 h the first day, then every 12–24 h during the hospitalization period.
After the procedure, a dual antiplatelet regimen of aspirin 100 mg and clopidogrel 75 mg daily for 3 months, after which 100 mg of aspirin daily was prescribed indefinitely.
1.3.10. Follow up
Follow up to the patients was done immediately after the procedure, during hospitalization, at 30 day and at 6 month duration.
1.4. Clinical follow up
For death (cardiovascular and non cardiovascular), bleeding, vascular complications, stoke, myocardial infarction, heart failure (NYHA functional class), conduction abnormalities and new onset AF.
1.5. Investigations
ECG follow up for ischemia, new onset AF and conduction abnormalities.
Echocardiographic follow-up for prosthesis function (peak and mean transvalvular gradient, peri- or intra-prosthetic leakage and other valve complication) as well as chamber size, function and severity of the mitral regurgitation.
1.5.1. Study endpoints
The primary end point of our study was device success as defined by the first Valve Academic Research Consortium consensus document, which is a technical composite end point including (1) successful vascular access, delivery, and deployment of the device and successful retrieval of the delivery system; (2) correct position of the device in the proper anatomical location; (3) good performance of the prosthetic heart valve (AVA > 1.2 cm2, mean aortic valve gradient <20 mmHg, or peak velocity <3 m/s, without moderate or severe prosthetic valve aortic regurgitation); and (4) only one valve implanted in the proper anatomical location.9
All end points and adverse events were predefined in accordance with the first Valve Academic Research Consortium consensus document.9
1.6. Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 20.0. Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage.
Paired sample t-test of significance was used when comparing between related sample.
Chi-square (X2) test of significance was used in order to compare proportions between two qualitative parameters.
Probability (p-value)
-
•
P-value < 0.05 was considered significant.
-
•
P-value < 0.001 was considered as highly significant.
-
•
P-value > 0.05 was considered insignificant.
2. Results
This multicenter, observational, retrospective and prospective study was conducted from February 2013 to March 2016 in Cairo, Egypt for 40 patients with severe symptomatic aortic stenosis and high risk for conventional surgical aortic valve replacement as predetermined according to the EUROSCORE II.
Transcatheter aortic valve implantation (TAVI) was done to all patients using either Core – valve revalving system or Sapien XT valve.
2.1. Baseline patients' characteristic data
The study included 40 patients with severe symptomatic AS, their age ranged from 68 years to 82 years with mean age is 73.98 ± 8.40. Twenty-one patients (52.5%) were males and 19 patients (47.5%) were females. Demographic data are demonstrated in Table 1.
Table 1.
Demographic data, risk factors and functional classification of the study population.
| Patients baseline data | ||
|---|---|---|
| Range | Mean ± SD | |
| Age (years) | 68–82 | 7 3.98 ± 8.40 |
| Gender | No. | % |
| Male | 21 | 52.5 |
| Female | 19 | 47.5 |
| Risk factors | No. | % |
| DM | 21 | 52.5 |
| HTN | 26 | 65.0 |
| PAD | 14 | 35.0 |
| COPD | 12 | 30.0 |
| History of cerebrovascular accidents | 4 | 10.0 |
| History of cancer | 2 | 5.0 |
| Smoking | 14 | 35.0 |
| History of ischemic heart disease | No. | % |
| SCAD | 23 | 57.5 |
| PCI | 9 | 22.5 |
| CABG | 9 | 22.5 |
| MI | 6 | 15.0 |
| Renal function(creatinine clearance ml/min) | No. | % |
| >60 ml/min | 33 | 82.5 |
| <60 ml/min | 6 | 15.0 |
| Dialysis | 1 | 2.5 |
| NYHA functional class | No. | % |
| NYHA I | 1 | 2.5 |
| NYHA II | 5 | 12.5 |
| NYHA III/IV | 34 | 85.0 |
Twenty-one patients (52.5%) were diabetics, 26 patients (65%) were hypertensive, 14 patients (35%) had peripheral arterial disease, 12 patients (30%) had COPD, 4 patients (10%) had history of cerebrovascular disease, 2 patients (5%) had history of malignancy, 14 patients (35%) were smokers.
Twenty-three patients (57.5%) had history of ischemic heart disease with stable coronary artery disease, 6 patients (15%) had history of MI, 9 patients (22.5%) had history of PCI and 9 patients (22.5%) underwent CABG Table 1.
Thirty-three patients (82.5) had creatinine clearance more than 60 ml/min, 6 patients (15%) had history of chronic kidney disease with creatinine clearance less than 60 ml/min but not on regular dialysis and 1 patient (2.5%) was already on regular dialysis Table 1.
All the patients had poor functional capacity with only one patients (2.5%) had NYHA I functional class, 5 patients (12.5) had NYHA functional class II while 34 patients (85.0%) had NYHA III/IV functional class Table 1.
2.2. Investigations
2.2.1. ECG
All patients had sinus rhythm on doing the TAVI, 6 patients (15%) had RBBB and 7 patients (17.5%) had LBBB Table 2.
Table 2.
preoperative investigations of the study population.
| Preoperative investigation | Range | Mean ± SD |
|---|---|---|
| ECG | No | % |
| Right bundle branch blockage | 6 | 15.0 |
| Left bundle branch block | 7 | 17.5 |
| Transthoracic Echocardiography | Range | Mean ± SD |
| EF (%) | 35–70 | 55.08 ± 9.71 |
| LVEDV (ml) | 78.3–119.4 | 108.88 ± 19.21 |
| LVESV (ml) | 36.2–68.3 | 44.08 ± 13.21 |
| LV mass index (gm/m2) | 110–244 | 157.93 ± 32.37 |
| Aortic valve | Range | Mean ± SD |
| Aortic valve area (cm2) | 0.45–1.0 | 0.75 ± 0.15 |
| Mean pressure gradient (mmHg) | 43–86 | 47.08 ± 11.08 |
| Maximum pressure gradient (mmHg) | 55–164 | 93.23 ± 18.69 |
| Aortic regurgitation | No | % |
| Non to trace (grade 0) | 10 | 25.0 |
| Mild (grade I) | 28 | 70.0 |
| Moderate (grade II) | 2 | 5.0 |
| Mitral regurgitation | No | % |
| Non to trace (grade 0) | 3 | 7.5 |
| Mild (grade I) | 11 | 27.5 |
| Moderate (grade II) | 25 | 62.5 |
| Severe (grade III and IV) | 1 | 2.5 |
| Annulus diameter | 20.6–25.8 | 21.9 ± 1.3 |
| Transesophageal Echocardiography | Range | Mean ± SD |
| Annulus diameter (mm) | 20.3–26.1 | 22.7 ± 1.8 |
| Diameter at the mid sinus level (mm) | 27.6–35.2 | 30.2 ± 3.1 |
| Preoperative MSCT | Range | Mean ± SD |
| Aortic annulus (mm) | 21.2–26.4 | 23.6 ± 2.1 |
| Height of coronary artery (mm) | Range | Mean ± SD |
| Left | 11.1–14.4 | 13.6 ± 2.1 |
| Right | 11.0–14.2 | 13.3 ± 1.5 |
| Common femoral artery diameter (mm) | Range | Mean ± SD |
| Right | 7.4–8.3 | 8.1 ± 1.5 |
| Left | 7.2–8.1 | 7.9 ± 1.4 |
2.2.2. Echocardiography
2.2.2.1. Transthoracic echocardiography
LV EF ranged from 35% to 70% with mean LVEF 55.08 ± 9.71, the LVEDV ranged from 78.3 to 119.4 ml with mean LVEDV 108.88 ± 19.21, the LVESV ranged from 36.2 to 68.3 ml with mean LVESV 44.08 ± 13.21 ml Table 2.
The LV mass index ranged from 110 gm/m2 to 244 gm/m2 with mean 157.93 ± 32.37 Table 2.
The aortic valve area ranged from 0.45 to 1.0 cm2 with mean valve area 0.75 ± 0.15, the mean pressure gradient across the aortic valve ranged from 43 mmHg to 86 mmHg with mean 47.08 ± 11.08 while the maximal pressure gradient ranged from 55 mmHg to 164 mmHg with mean 93.23 ± 18.69 Table 2.
Pre operatively, 10 patients (25.0%) had non to trace (grade 0) aortic valve regurgitation, while 28 patients (70.0%) had mild (grade I) AR, 2 patients (5.0%) had moderate (grade II) AR and no patients had severe (grade III and IV) AR Table 2.
Three patients (7.5%) had non to trace (grade 0) mitral valve regurgitation, 11 patients (27.5%) had mild (grade I) MR, 25 patients (62.5%) had moderate (grade II) MR and one patient (2.5%) had severe (grade III and IV) MR.
The aortic annulus measured by transthoracic echo ranged from 20.6 to 25.8 mm with mean annulus diameter 21.9 ± 1.3.
2.2.2.2. Transesophageal echocardiography
The aortic annulus measured by transesophageal echo ranged from 20.3 to 26.1 with mean annulus diameter 22.7 ± 1.8. The diameter at the mid sinus level ranged from 27.6 to 35.2 mm with mean value 30.2 ± 3.1 (Table 2).
MSC T Preoperative MSCT showed that the aortic annulus measured by MSCT ranged from 21.2 to 26.4 with mean annulus diameter 23.6 ± 2.1, the height of the left coronary artery ranged from 11.1 to 14.4 mm with mean of 13.6 ± 2.1. The height of the left coronary artery ranged from 11.0 to 14.2 mm with mean of 13.3 ± 1.5 (Table 2).
The right common femoral artery diameter ranged from 7.4 to 8.3 mm with mean of 8.1 ± 1.5, the left common femoral artery diameter ranged from 7.2–8.1 mm with mean of 7.9 ± 1.4 (Table 2).
2.3. Procedural outcome
In 17 patients (42.5%) we used the Sapien XT valve. In 23 patients (57.5%) we used the Core Valve revalving system. In one patient we used 2 valves, size 29 and 31 (CoreValve, valve in valve) due to moderately severe paravalvular regurgitation Table 3.
Table 3.
valves used for TAVI in the study.
| Valve type | Total No | % | Size | No | % |
|---|---|---|---|---|---|
| Sapien XT valve | 17 | 42.5 | 23 | 7 | 17.5 |
| 26 | 10 | 25.0 | |||
| Core valve | 23 | 57.5 | 26 | 16 | 40.0 |
| 29 | 6 | 15.0 | |||
| 31 | 1 | 2.5 | |||
Procedural success was achieved in 39 patients (97.5%). No mortality occurred during the procedure. One patient (2.5%) developed cerebrovascular stroke, no patients developed myocardial infarctions.
Minor bleeding occurred in 5 patients (12.5%) while major bleeding occurred in 2 patients (5.0%) and no patients has been developed life threatening bleeding Table 4.
Table 4.
Procedural outcome.
| Procedural outcome | No. | % |
|---|---|---|
| Success | 39 | 97.5 |
| Death | 0 | 0.0 |
| CVA | 1 | 2.5 |
| MI | 0 | 0.0 |
| Bleeding | ||
| Minor | 5 | 12.5 |
| Major | 2 | 5.0 |
| Life threatening | 0 | 0.0 |
| Vascular complications | ||
| Minor | 3 | 7.5 |
| Major | 2 | 5.0 |
| Permanent pacemaker | 1 | 2.5 |
| New AF | 0 | 0.0 |
| Migration | 0 | 0.0 |
| Surgery | 0 | 0.0 |
| Valve in valve | 1 | 2.5 |
| Prosthetic regurgitation | ||
| Non to trace | 16 | 40.0 |
| Mild | 22 | 55.0 |
| Moderate | 2 | 5.0 |
| Severe | 0 | 0.0 |
Minor vascular complication occurred in 3 patients (7.5%) while major vascular complication occurred in 2 patients (5.0%). No patients developed new AF, one permanent pacemaker inserted for one patient (2.5%) during TAVI, no valve migration occurred and no patients referred urgently for surgery Table 4.
None to trace (grade 0) prosthetic aortic regurgitation was found in 16 patients (40.0%), 22 patients (55.0%) had mild (grade I) prosthetic aortic regurgitation, 2 patients (5.0%) had moderate prosthetic aortic regurgitation (grade II) and no patients had severe (grade III and IV) prosthetic aortic regurgitation Table 4.
2.4. In-hospital outcome
During the hospitalization period, no patients died, only one patient (2.5%) developed cerebrovascular stroke while no one developed acute myocardial infarction Table 5.
Table 5.
in hospital outcome among the study group.
| No. | % | |
|---|---|---|
| Death | 0 | 0.0 |
| CVA | 1 | 2.5 |
| MI | 0 | 0.0 |
| New onset AF | 3 | 7.5 |
| PPM | 4 | 10.0 |
| Bleeding | ||
| Minor | 1 | 2.5 |
| Major | 1 | 2.5 |
| Life threatening | 0 | 0.0 |
| Vascular complication | ||
| Minor | 1 | 2.5 |
| Major | 1 | 2.5 |
Three patients (7.5%) developed recent atrial fibrillation, one patient developed AF immediately after the procedure and the other 2 patients late during the in-hospital period. Permanent pacemaker was inserted for 4 patients (10%) due to complete heart block, 3 patients with the Core Valve and 1 patient with the Sapien XT valve.
Minor bleeding occurred in 1 patient (2.5%), major bleeding occurred in another one patient (2.5%) while no patients developed life threatening bleeding. One patient (2.5%) developed minor vascular complications and another one patient (2.5%) developed major vascular complication Table 5.
2.4.1. Days outcome
One patient (2.5%) died due to a cardiovascular complication (MI) while another one died due to non cardiovascular cause (renal failure). Myocardial infarction occurred in one patient (2.5%). One more patient (2.5%) developed new onset AF. Re hospitalization was done for one patient (2.5%) due to heart failure Table 6.
Table 6.
Thirty day outcome among the study group.
| No. | % | |
|---|---|---|
| Death (Total) | 2 | 5.0 |
| Any | 1 | 2.5 |
| CV | 1 | 2.5 |
| CVA | 0 | 0.0 |
| MI | 1 | 2.5 |
| New AF | 1 | 2.5 |
| PPM | 0 | 0.0 |
| Re hospitalization | 1 | 2.5 |
2.5. Months outcome
One patient 1/38 (2.6%) died due to non cardiovascular cause (cancer head of pancreas). Re hospitalization was done for one patient 1/38 (2.6%) due to heart failure. None to trace (grade 0) paraalvular prosthetic aortic regurgitation was found in 20/38 patients (52.6%), 17/38 patients (44.7%) had mild (grade I) paraalvular prosthetic aortic regurgitation, 1/38 patients (2.6%) had moderate (grade II) and no patients had severe (grade III and grade IV) paraalvular prosthetic aortic regurgitation Table 7.
Table 7.
Outcome at 6 month among the study population.
| Death | No. | % |
|---|---|---|
| Total | 1/38 | 2.6 |
| Any | 1/38 | 2.6 |
| CV | 0 | 0.0 |
| CVA | 0 | 0.0 |
| MI | 0 | 0.0 |
| New AF | 0 | 0.0 |
| PPM | 0 | 0.0 |
| Migration | 0 | 0.0 |
| Thrombosis | 0 | 0.0 |
| Surgery | 0 | 0.0 |
| Re hospitalization | 1/38 | 2.6 |
| Prosthetic regurgitation | No. | % |
| Non to trace | 20/38 | 52.6 |
| Mild | 17/38 | 44.7 |
| Moderate | 1/38 | 2.6 |
2.5.1. End study outcome
At the end of the study, 3 patients died (7.5%), cerebrovascular accident occurred in 2 patients (5.0%), MI occurred in one patient (2.5%), minor bleeding occurred in 6 patients (15.0%), major bleeding occurred in 3 patients (7.5%). Minor vascular complications occurred in 4 patients (10.0%) while major vascular complications occurred in 3 patients (7.5%). New onset AF occurred in 4 patients (10.0%). Permanent pacemaker insertion was done for 5 patients (12.5%). Both aortic and mitral regurgitation was significantly improved with improvement of NYHA functional class.
2.6. Functional class improvement after TAVI
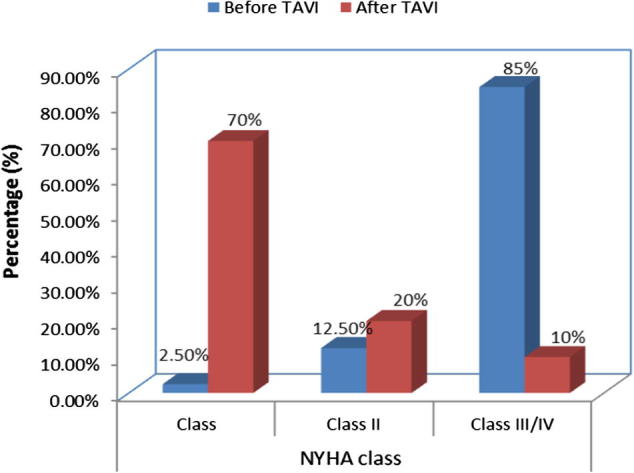
One patients (2.5%) was in NYHA functional class I before TAVI while 28 patients (70.0%) found with functional class I after TAVI (p < 0.001). 5 patients (12.5%) was in class II before TAVI while 8 patients (20.0%) was found with class II after TAVI (p 0.544). 34 patients (85%) was in NYHA class III/IV before TAVI and 4 patients (10.0%) had the same class after TAVI (p < 0.001) Table 8, Fig. 1.
Table 8.
Comparison between before and after TAVI.
| Variables |
Before TAVI |
After TAVI |
P value | ||
|---|---|---|---|---|---|
| NYHA functional class | No. | % | No. | % | |
| Class I | 1 | 2.5 | 28 | 70.0 | <0.001 |
| Class II | 5 | 12,5 | 8 | 20.0 | 0.544 |
| Class III/IV | 34 | 85.0 | 4 | 10.0 | <0.001 |
| Echo measurements | Range | Mean ± SD | Range | Mean ± SD | |
| EF(%) | 35–70 | 55.08 ± 9.71 | 39–73 | 58.88 ± 8.79 | <0.001 |
| EDV (ml) | 78.3–119.4 | 108.88 ± 19.21 | 56.6–104.7 | 92.96 ± 16.97 | <0.001 |
| ESV (ml) | 36.2–68.3 | 44.08 ± 13.21 | 33.5–51.2 | 41.41 ± 14.33 | 0.036 |
| Mass index | 110–244 | 157.93 ± 32.37 | 88–186 | 133.50 ± 21.96 | <0.001 |
| Valve area (cm2) | 0.45–1.0 | 0.75 ± 0.15 | 1.52–2.4 | 1.96 ± 0.18 | <0.001 |
| Mean PG (mmHg) | 43–86 | 47.08 ± 11.08 | 3–20 | 10.28 ± 3.21 | <0.001 |
| Max PG (mmHg) | 55–164 | 93.23 ± 18.69 | 5–36 | 20.01 ± 5.92 | <0.001 |
| PAP (mmHg) | 21–97 | 45.30 ± 16.88 | 23–66 | 38.98 ± 10.93 | <0.016 |
| Aortic regurgitation | No. | % | No. | % | |
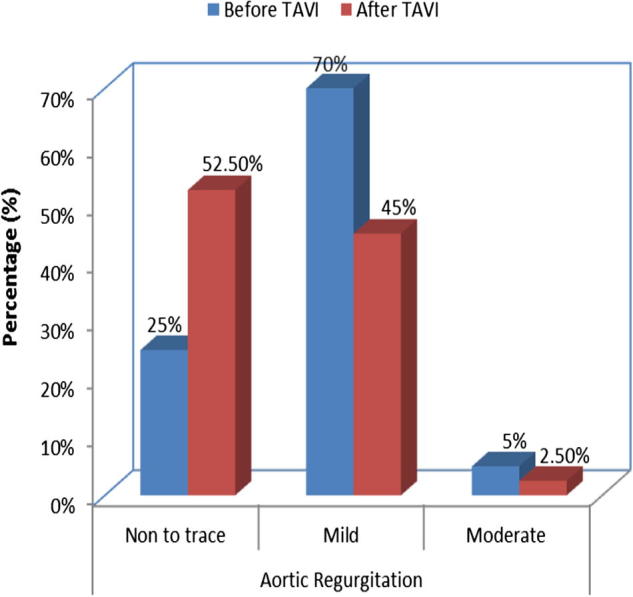
| Non to trace | 10 | 25.0 | 21 | 52.5 | 0.022 |
| Mild | 28 | 70.0 | 18 | 45.0 | 0.042 |
| Moderate | 2 | 5.0 | 1 | 2.5 | 0.82 |
| Mitral regurgitation | No. | % | No. | % | |
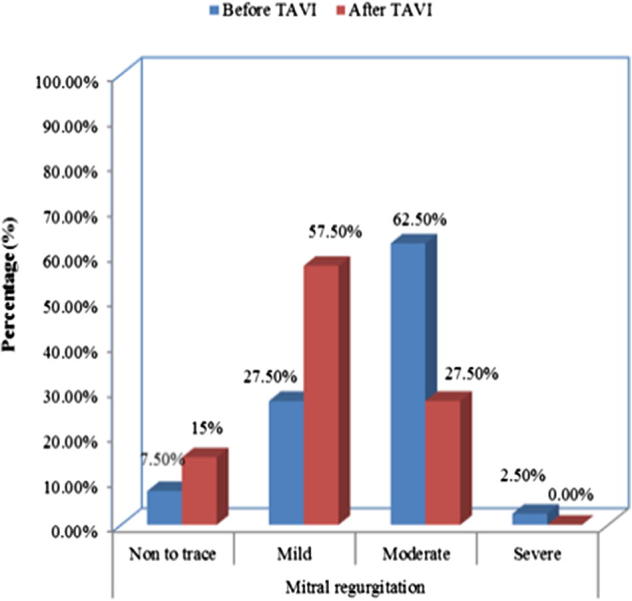
| Non to trace | 3 | 7.5 | 6 | 15 | 0.47 |
| Mild | 11 | 27.5 | 23 | 57.5 | 0.013 |
| Moderate | 25 | 62.5 | 11 | 27.5 | 0.004 |
| Severe | 1 | 2.5 | 0 | 0.0 | 0.909 |
Fig. 1.
NYHA functional class before and after TAVI.
2.7. Echocardiographic difference between before and after TAVI
The mean left ventricular ejection fraction was 55.08 ± 9.71 before TAVI and increased to 58.88 ± 8.79 after TAVI (p < 0.001). The mean left ventricular end diastolic volume was 108.88 ± 19.21 before TAVI and was 92.96 ± 16.97 after TAVI (p < 0.001). The mean left ventricular end systolic volume was 44.08 ± 13.21 before TAVI and was 33.41 ± 14.33 after TAVI (p = 0.036) Table 8 (see Table 9).
Table 9.
Comparison between the Core Valve and SAPIEN XT valves.
| Core valve no /% | SAPIEN XT no/% | P value | |
|---|---|---|---|
| Procedural and immediate outcome | |||
| Procedural success | 22/23 (95.7%) | 17/17 (100%) | 0.869 |
| Second valve | 1/23(4.3%) | 0/17(0.0%) | 0.866 |
| Valve migration | 0/23(0.0%) | 0/17(0.0%) | 1.0 |
| Referral for urgent surgery | 0/23(0.0%) | 0/17(0.0%) | 1.0 |
| Procedural death | 0/23(0.0%) | 0/17(0.0%) | 1.0 |
| Stroke | 1/23(4.3%) | 0/17(0.0%) | 0.866 |
| Bleeding | |||
| Minor | 3/23 (13.0%) | 3/17 (17.6%) | 0.964 |
| Major | 1/23 (4.3%) | 2/17 (11.6%) | 0.794 |
| Life threatening | 0/23 (0.0%) | 0/17 (0.0%) | 1.000 |
| Vascular complications | |||
| Minor | 2/23 (8.6%) | 2/17 (11.6%) | 0.825 |
| Major | 2/23 (8.6%) | 1/17 (5.8%) | 0.782 |
| At 6 month outcome | |||
| Mortality (total) | 2/23 (8.6%) | 1/17 (5.8%) | 0.782 |
| Cardiac | 1/23 (4.3%) | 0/17 (0.0%) | 0.866 |
| Non cardiac | 1/23 (4.3%) | 1/17 (5.8%) | 0.602 |
| Stoke | 1/23 (4.3%) | 1/17 (5.8%) | 0.602 |
| MI | 1/23 (4.3%) | 0/17 (0.0%) | 0.866 |
| New onset AF | 2/23 (8.6%) | 2/17 (11.6%) | 0.825 |
| Permanent pacemaker | 4/23 (17.2%) | 1/17 (5.8%) | 0.550 |
| Paravalvular regurgitation | |||
| Non to trace (grade 0) | 11/23 (47.85%) | 10/17 (58.9%) | 0.708 |
| Mild (grade I) | 11/23 (47.85%) | 7/17 (41.1%) | 0.918 |
| Moderate (grade II) | 1/23 (4.3%) | 0/17 (0.0%) | 0.866 |
| Severe (grade III and IV) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Rehospitalization | 1/23 (4.3%) | 1/17 (5.8%) | 0.602 |
The mean left ventricular mass index was 157.93 ± 32.37 before TAVI and was 133.50 ± 21.96 after TAVI (p < 0.001).
The mean aortic valve area was 0.75 ± 0.15 before TAVI and 1.96 ± 0.18 after TAVI (p < 0.001).
The mean of the maximal and mean pressure gradient over the aortic valve was significantly decreased after TAVI (p < 0.001) Table 8.
The mean pulmonary arterial systolic pressure was 45.30 ± 16.88 before TAVI and was 38.98 ± 10.93 after TAVI (p = 0.016) Table 8.
Ten patients (25.0%) had non to trace (grade 0) AR before TAVI while 21 patients (52.5%) had non to trace (grade 0) AR after TAVI (p = 0.022). Twenty-eight patients (70.0%) had mild (grade I) AR before TAVI while 18 patients (45%) had mild (grade I) AR after TAVI (p 0.042). Two patients (5.0%) had moderate (grade II) AR before TAVI and only one patient (2.5%) had moderate (grade II) AR after TAVI (p = 0.8). No patients had severe (grade III and IV) AR neither before nor after TAVI Table 8 and Fig. 2.
Fig. 2.
Grades of MR before and after TAVI.
Three patients (7.5%) had non to trace (grade 0) MR before TAVI while 6 patients (15%) had non to trace (grade 0) MR after TAVI (p = 0.4). Eleven patients (27.5%) had mild (grade I) MR before TAVI while 23 patients (57.5%) had mild (grade I) MR after TAVI (p = 0.013). Twenty-five patients (62.5%) had moderate (grade II) MR before TAVI while 11 patients (27.5%) had moderate (grade II) MR after TAVI (p = 0.004). One patient (2.5%) had severe (grade III and IV) MR before TAVI while no patient had severe (grade III and IV) MR after TAVI (p = 0.9) Table 8 and Fig. 3.
Fig. 3.
Grades of AR before and after TAVI.
2.8. Subgroup analysis
2.8.1. Procedural and immediate post procedural outcome
In 23 patients (57.5%) we used CoreValve and for 17 patients (42.5) we used the Sapien XT valve.
Procedural success was 100% for all Sapien XT valve and 95.7% for the CoreValve (p = 0.8) as in one patient (4.3%) we need to implant another valve during the procedure due to moderately severe paravalvular regurgitation in the Core Valve group while no second valve needed in the Sapien XT group (p = 0.8).
Cerebrovascular stroke occurred in one patient in subgroup I while no stroke occurred in the sub class II (p = 0.866).
Minor bleeding occurred in 3 patients (13.0%) in the Core Valve group, and in 3 patient (17.6%) in the SAPIEN XT group (p = 0.9). Major bleeding occurred in 1 patient (4.3%) in the Core Valve group, and in 2 patient (11.6%) in the Sapien XT group (p = 0.7) while life threatening bleeding not occurred in both groups.
Minor vascular complications occurred in 2 patients (8.6%) in the Core Valve group and in 2 patients (11.6%) in the Sapien XT group. (p = 0.8) Major vascular complications occurred in 2 patients (8.6%) in the Core Valve group and in 1 patient (5.8%) in the Sapien XT group (p = 0.7).
One patient (4.3%) in the Core Valve group underwent pacemaker implantation during TAVI due to CHB.
2.8.2. At 6 months outcome
At 6 months death occurred in 2 patients (8.6%) in the Core Valve group, one due to cardiac cause and the other one due to non cardiovascular cause while death occurred in 1 patient (5.8%) in the Sapien XT group due to non cardiovascular cause (p = 0.7).
Stroke occurred in 1 patient (4.3%) in the Core Valve group and in 1 patient (5.8%) in the Sapien XT group (p = 0.6).
Myocardial infarction occurred in 1 patient (4.3%) in the Core Valve group while no patients developed MI in the Sapien XT group (p = 0.8).
New onset AF occurred in 2 patients (8.6%) in the Core Valve group and in 2 patients (11.6%) in the Sapien XT group (p = 0.8).
Permanent pacemaker insertion was done for 4 patients (17.2%) in the Core Valve group (including one pacemaker implanted during TAVI) and in one patient (5.8%) in the Sapien XT group (p = 0.55).
Non to trace (grade 0) paravalvular regurgitation was found in 11/23 patients (47.85%) in the Core Valve group while it was found in 10/17 patients (58.9%) in the Sapien XT group (p = 0.7). Mild (grade I) paravalvular regurgitation was found in 11/23 patients (47.85%) in the Core Valve group while it was found in 7/17 patients (41.1%) in the Sapien XT group (p = 0.918). Moderate (grade II) paravalvular regurgitation was found in 1/23 patient (4.3%) in the Core Valve group while no patients developed moderate (grade II) paravalvular regurgitation in the Sapien XT group (p = 0.8). No patients in both groups had severe paravalvular aortic regurgitation (grade III and IV).
Re hospitalization was done for 1 patient (4.3%) in the Core Valve group, and in 1 patient (5.8%) in the Sapien XT group (p = 0.6).
3. Discussion
The transcatheter aortic valve implantation is relatively a new entity in the field of the interventional cardiology but it gains more space in the past two decades and the improving results and learning curve allowed more use of TAVI for patients with severe symptomatic aortic stenosis and high risk for standard surgical aortic valve replacement.5
Improving the valve profile of the two commercially available valves with new generation of the Edward’s valve (Sapien III) and the new version of the Core Valve (The CoreValve Evolut R with EnVeo R delivery catheter) also reduce the complications especially the vascular complications and subsequent bleeding, both reduce the total and cardiovascular mortality.
Our study was conducted on 40 patients, they are relatively old with a mean age of 73.98 ± 8.40 years with EuroSCORE ranged from 15% to 21% with mean 18.1 ± 1.96% and this high EuroSCORE is expected as our patients are elderly and had multiple comorbidities.
Device success, cardiovascular mortality, myocardial infarction, stroke, bleeding, and vascular complications were defined according to Valve Academic Research Consortium (VARC) definitions.9
3.1. Procedural and immediate post procedural outcome
In the current study Procedural success was achieved in 39 patients (97.5%) that appear to be comparable with other published series, such as the Italian registry (98%),10 the French registry (92.6%),11 the Belgian registry (97%),12 the German registry (98.4%),13 the European SOURCE registry (93.8%)14 and the Canadian experience (93.3%).15 In the first Egyptian experience of TAVI trial that was done for 10 patients who received the Edward’s Sapien valve and Sapien XT valve and showed success rate of 100%.16
We used a second valve in only one patient (2.5%) as we implant another valve (Core valve with size 31) due to moderately severe paravalvular leak with the first one (Core valve with size 29). In the rest of patients we used only one valve per patient and this was comparable to that reported in other trials.
In PARTENER trial the incidence of second transcatheter valve was 2.2% because of moderate or severe aortic regurgitation.5 The incidence was 2.3% in the France 2 registry,17 while it was 0.4% in the PARTENER II trial.18
In the present study minor bleeding occurred in 6 patients (15.0%), major bleeding occurred in 3 patients (7.5%) while life threatening bleeding not occurred at all and it was comparable to that reported in the PARTNER trial, the rates of major bleeding was 9.3% in cohort B and 16.8% in cohort A).5
The France 2 registry reported 2.7% incidence of life-threatening or major bleeding in patients who underwent the transfemoral procedure.17
In our study minor vascular complications occurred in 4 patients (10.0%) while major vascular complications occurred in 3 patients (7.5%) and it was comparable with results of other studies. In the PARTENER II trial major vascular complications were reported in 7.9% and 8.4% of patients at 30 days and 1 year respectively.18 In the France 2 registry, major vascular complications were reported in 4.7% of patients.17
In the present study; cerebrovascular stroke occurred in 2 patients (5.0%), one stroke occurred during the procedure and the other during the hospitalization period and both are ischemic strokes, this incidence is comparable to other studies. In the France 2 registry the incidence of stroke was (4.1%)17 In PARTENER trial major strokes were 5.0% at 30 days and 7.8 at 1 year.5
In the CHOICE trial, the incidence of stroke was 5.8% in the balloon-expandable valve group and 2.6% in the self-expandable valve group.19
In the PARTENER II trial the incidence of stroke was 5.5% at 30 days and 8.0% at 1 year.18
In the First Egyptian experience of Transcatheter Aortic Valve Implantation trial the incidence of TIAs occurred in one patient (10%).16
3.2. At 6 month outcome
Total mortality in our study was 7.5% (3 patients), one patient died due to a cardiovascular cause, he devolved acute MI not related to the device, the other 2 patient died due to non cardiovascular causes, this was comparable to other studies. In PARTENER II trial all causes mortality was 3.9% at 30 days and 12.5% at 1 year.18
In the France 2 registry the 30 days mortality from all causes was 9.7%.17
In the CHOICE trial the 30 days all causes mortality was 4.6% and it reported all causes mortality 4.1% in the balloon-expandable group and 5.1% in the self-expandable valve group.19
New onset atrial fibrillation occurred in 4 patients (10.0%), and it was comparable with results of PARTENER 2 trial that showed an incidence of new AF at 30 day and one year are 9.1% and 10.1% respectively.18
CHB that mandate implantation of the permanent pacemaker was found in 5 patients (12.5%) and this was comparable to that reported in other registries
In France 2 registry; the incidence of permanent pacemaker implantation was 15.6%,17 13% in Belgium registry,12 16.3% in the United Kingdom registry,20 16.6% in Italy,21 and 6.7% in SOURCE.14
In the PARTENER 2 trial, the incidence of new pacemaker insertion was 8.5% at 30 days and 9.9% at 1 year.18
In the First Egyptian experience of Transcatheter Aortic Valve Implantation trial permanent pacemaker insertion was done for one patient (10%).16
In our study the aortic valve area is significantly increased after TAVI. The mean aortic valve area was 0.75 ± 0.15 before TAVI and 1.96 ± 0.18 after TAVI (p < 0.001), this was comparable to the result of first Egyptian experience of TAVI with post procedural AVA 2.0 ± 0.1,16 the PARTENER II trial that showed increased mean AVA from 0.6 ± 0.2 cm2 before TAVI to 1.5 ± 0.4 at 30 days and to 1.6 ± 0.5 at I year.18
In the present study the mean pressure gradient across the aortic valve was significantly decreased from 47.08 ± 11.08 mmHg before TAVI to 10.28 ± 3.21 mmHg after TAVI (p < 0.001) and this was comparable to that reported in the PARTENER II trial that showed the mean pressure gradient was decreased from 44.7 ± 15.4 mmHg before TAVI to 11.4 ± 7.0 mmHg at 30 days and 13.2 ± 11.2 mmHg 1 year after TAVI (p < 0.001).18
The incidence of moderate aortic regurgitation in our study was 2.5%, while most of the patients either had non to trace paravalvular aortic regurgitation (52.5%) or mild paravalvular aortic regurgitation (45%). No patients developed severe AR. In PARTENER trial, moderate or severe paravalvular aortic regurgitation was present in 11.8% of the patients in the TAVI group at 30 days and in 10.5% at 1 year.5
In the France 2 registry the incidence of paravalvular regurgitation was 46.0% for grade 1 regurgitation, 16.1% for grade 2 and 0.8% for grade 3 paravalvular regurgitation.17
The small number of patients in our study may explain such difference.
There were significant improvement of LVEF, the mean LVEF increased from 55.08 ± 9.71 before TAVI to 58.88 ± 8.79 after TAVI (p < 0.001) and this was comparable with PARTENER II trial that showed improvement of mean EF from 53.9 ± 13.1 to 57.9 ± 10.1 at 30 days and 57.2 ± 10.6 at 1 year (p < 0.001).18
After TAVI significant improvement of the NYHA functional class was noted, 34 patients (70%) was in class III/IV before TAVI while 4 (10.0%) become in functional class III/IV after TAVI (p < 0.001).
A recent New Zealand study found that symptom class improved significantly, pre-operatively 72% of patients had NYHA class III–IV compared with 20% at 30 days.22
In the PARTNER trial, TAVI resulted in lower rates of NYHA III–IV symptoms at 1 year when compared to medical therapy (23.7% vs 50.0%, p < 0.001).5
In our study the incidence of rehospitalization was 5.0% and all patients are re hospitalized due to heart failure and it was comparable to that reported in the CHOICE trial in which the rehospitalization rate was 4.3% at 30 days in the self-expandable valve group while no rehospitalization in the balloon expandable valve group.19
3.3. Subgroup analysis
In subgroup analysis we compare both type of valves used in our study as regard procedural and 6 month outcome.
3.3.1. Procedural and immediate post procedural outcome
In the present study; we used the CoreValve in 23 patients (57.5%) and in 17 patients (42.5%) we used the Sapien XT valve. Procedural success was 95.7% for the CoreValve and 100% for Sapien XT valve (p = 0.8). In the CHOICE trial, the first randomized clinical trial that compared the 2 different transcatheter heart valve technologies in high-risk patients with severe aortic stenosis, the procedural success was significantly higher in the balloon-expandable valve group (95.9%) than in the self-expandable valve group (77.5) (p < 0.001).19
The implantation of the second valve was done for one patient 1/23 (4.3%) in the Core Valve group as in one patient we need to implant another valve due to moderately severe paravalvular regurgitation while such second valve not done for those in the Sapien XT group (p = 0.8).
Results of the CHOICE trial showed that implantation of a second valve (transcatheter valve-in-valve) was also less frequent in the balloon-expandable group (1 patient, 0.8%) than the self-expandable valve group (7 patients, 5.8%, p = 0.03).19
In our study such difference may be attributed to unequal numbers of patients in the 2 groups and small sample size.
In the present study; no procedural mortality was reported for both valves and this was similar to that reported in the CHOICE trial.19
Also no valve migration occurred with both type of valves and no patients referred urgently for surgery.
Minor bleeding occurred in 3/23 patients (13%) in the Core Valve group, and in 3/17 patients (17.6%) in the Sapien XT group (p = 0.9). Major bleeding occurred in 1/23 patient (4.3%) in the Core Valve group, and in 2/17 patients (11.6%) in the Sapien XT group (p = 0.7), while life threatening bleeding not occurred in both groups (p = 0.7).
In the CHOICE trial the incidence of minor bleeding was 7.7% for Core Valve and 9.1% for Sapien XT valve (p = 0.7), the incidence of major bleeding was 14.5% for Core Valve and 19.0% for Sapien XT valve (p = 0.36).19
Minor vascular complications occurred in 2/23 patients (8.6%) in the Core Valve group and in 2/17 patients (11.6%) in the Sapien XT group (p = 0.8). Major vascular complications occurred in 2/23 patients (8.6%) in the Core Valve group and in 1/17 patient (5.8%) in the Sapien XT group (p = 0.78). In the CHOICE trial the incidence of minor vascular complications was 4.1% for Core Valve and 1.7% for Sapien XT valve (p = 0.28), the incidence of major vascular complications was 11.1% for Core Valve and 9.9% for Sapien XT valve (p = 0.76).19
Stroke occurred in only one patient 1/23 (4.3%) with the Core Valve during the implantation procedure, and also one patient 1/17 (5.8%) from the Sapien group developed stroke during the hospitalization period (p = 0.602).
In the CHOICE trial the incidence of stroke was 2.6% in the self-expandable valve group and 5.8% in the balloon-expandable valve group (p = 0.3),19 again this difference may be attributed to unequal numbers of patients in the 2 groups and small sample size.
3.3.2. At 6 month outcome
At 6 months, death occurred in 2/23 patients (8.6%) in the Core Valve group and in 1/17 patient (5.8%) in the Sapien XT group (p = 0.78) and this was comparable to that of the CHOICE trial in which the incidence of 30 days mortality was 4.1% in the balloon-expandable group and 5.1% in the self-expandable valve group (p = 0.77).19
At 6 months, myocardial infarction occurred in 1/23 patient (4.3%) in the Core Valve group while no patients developed MI in the Sapien XT group (p = 0.8). In the CHOICE trial the incidence of MI was 0.8% for the Sapien XT valve group and not occurred with the Core Valve group (p = 0.99).19
New onset AF occurred in 2 patients (8.6%) in the Core Valve group, and in 2 patients (11.6%) in the Sapien XT group (p = 0.8).
At 6 months, permanent pacemaker insertion was done for 4/23 patients (17.2%) in the Core Valve group and in 1/17 patient (5.8%) in the Sapien XT group (p = 0.55). In the CHOICE trial, implantation of a new permanent pacemaker was lower in the balloon-expandable valve group (p = 0.001).19 In France 2 registry new, permanent pacemaker was required more often in patients receiving CoreValve devices than in those receiving Sapien XT devices (24.2% vs 11.5%).17
In our study, despite low incidence of permanent pacemaker insertion with the Sapien XT valve but it is not statistically significant due to unequal number of patients and small sample size of our study.
Non to trace (grade 0) paravalvular aortic regurgitation occurred in 11/23 patients (47.85%) for the Core Valve group and 10/17 patients (58.9%) for the Sapien XT group (p = 0.7). Mild (grade 0) paravalvular aortic regurgitation occurred in 11/23 patients (47.85%) for the Core Valve group and 7/17 patients (41.1%) for the Sapien XT group (p = 0.9). Moderate (grade 2) paravalvular aortic regurgitation occurred in 1/23 patient (4.3%) for the Core Valve group while no patients developed moderate (grade 2) paravalvular aortic regurgitation in the Sapien XT group (p = 0.866). No patients in our study developed severe paravalvular regurgitation.
In the CHOICE trial, non to trace (grade 0) paravalvular aortic regurgitation occurred in 59/120 patients (49.2%) for the Core Valve group and 88/121 patients (66.1%) for the Sapien XT group. Mild (grade 1) paravalvular aortic regurgitation occurred in 54/120 patients (45.0%) for the Core Valve group and 39/121 patients (32.2%) for the Sapien XT group (p = 0.8). Moderate (grade 2) paravalvular aortic regurgitation occurred in 7/120 patients (5.8%) for the Core Valve group and 1/121 patients (0.8%) for the Sapien XT group. Severe (grade 3) paravalvular aortic regurgitation occurred 1/121 patients (0.8%) for the Sapien XT group but not occurred in the Core Valve group.19
Results of our study were similar to that of the CHOICE trial in the incidence of paravalvular regurgitation for grade o, grade 1 and different as regard grade 3 and grade 4 and this may be explained by small number of patient in our study.
In our study the incidence of re hospitalization was 5.0% and all patients are re hospitalized due to heart failure, 1/23 patient (4.3%) in the Core Valve group and also 1/17 patient (5.8%) in the Sapien XT group while in the CHOICE trial the incidence of rehospitalization was 4.3% at 30 days in the self-expandable valve group while no rehospitalization in the balloon expandable valve group.19
3.4. Conclusion
TAVI is a safe and effective procedure in selected high-risk patients with severe symptomatic aortic stenosis and there is no significant difference between used valves.
3.5. Limitation
Our study has all the limitations inherent to a relatively small, retrospective study. The small number of the patients skews the complication rate adversely and affects the statistical significance of the study outcomes. Also the follow up period is short and more late complications cannot be addressed well.
Acknowledgments
Conflict of interest
We have no conflict of interest.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
Contributor Information
Hamdy Soliman, Email: helliwa717@gmail.com.
Shaimaa Mostafa, Email: shaimaamustafa2011@gmail.com.
References
- 1.Lung B., Baron G., Butchart E.G. The Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Euro J Cardio-Thoracic Surg: Off J Euro Assoc Cardio-Thoracic Surg. 2012;42:S1-44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 3.Picano E., Pibarot P., Lancellotti P., Monin J.L., Bonow R.O. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am College Cardiol. 2009;54:2251–2260. doi: 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Kolh P., Kerzmann A., Honore C., Comte L., Limet R. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Euro J Cardio-Thoracic Surg. 2007;31:600–606. doi: 10.1016/j.ejcts.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Smith C.R., Leon M.B., Mack M.J. PARTNER trial investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Otto C.M. Aortic stenosis and hyperlipidemia: establishing a cause-effect relationship. Am Heart J. 2004;147:761–763. doi: 10.1016/j.ahj.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Chamber quantification writing group; American society of echocardiography's guidelines and standards committee; European association of echocardiography. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H., Hung J., Bermejo J. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:5. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Leon M.B., Piazza N., Nikolsky E. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–217. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamburino C., Capodanno D., Ramondo A. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 11.Eltchaninoff H., Prat A., Gilard M. Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 12.Bosmans J.M., Kefer J., De Bruyne B. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12:762–767. doi: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- 13.Zahn R., Gerckens U., Grube E. First results from a multi-centre real-world registry. Eur Heart J. 2011;32 doi: 10.1093/eurheartj/ehq339. 198–198. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M., Schymik G., Walther T. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) registry: a european registry of transcatheter aortic valve implantation using the Edwards Sapien valve. Circulation. 2010;122:62. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 15.Rodes C.J., Webb J.G., Cheung A. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Khashaba A., Adel W., Roshdi A., Gafar A., Essam S., Algendy M. First Egyptian experience of transcatheter aortic valve implantation: immediate results and one year follow up. Egypt Heart J. 2014;66:17–21. [Google Scholar]
- 17.Martine G., Eltchaninoff H., Iung B. The FRANCE 2 investigators registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 18.Martin B., Leon M.B., Smith C.R. For the PARTNER 2 investigators transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:17. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Wahab M., Mehilli J., Frerker C. The CHOICE investigators Comparison of Balloon-Expandable vs Self-expandable Valves in Patients Undergoing Transcatheter Aortic Valve Replacement. CHOICE Rand Clin Trial JAMA. 2014;311:1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 20.Moat N.E., Ludman P., deBelder M.A. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Kahlert P., Erbel R. Transcatheter aortic valve implantation in the era after commercialization: quo vadis in the real world? Circulation. 2011;123:239–241. doi: 10.1161/CIRCULATIONAHA.110.004713. [DOI] [PubMed] [Google Scholar]
- 22.Sylvia Y.W., Tom K.M.W., Parma N. Early outcome of patients undergoing transcatheter aortic valve implantation (TAVI): the Auckland City Hospital experience. New Zealand Med J. 2016;1428:2011–2015. [PubMed] [Google Scholar]


