Fig. 4.
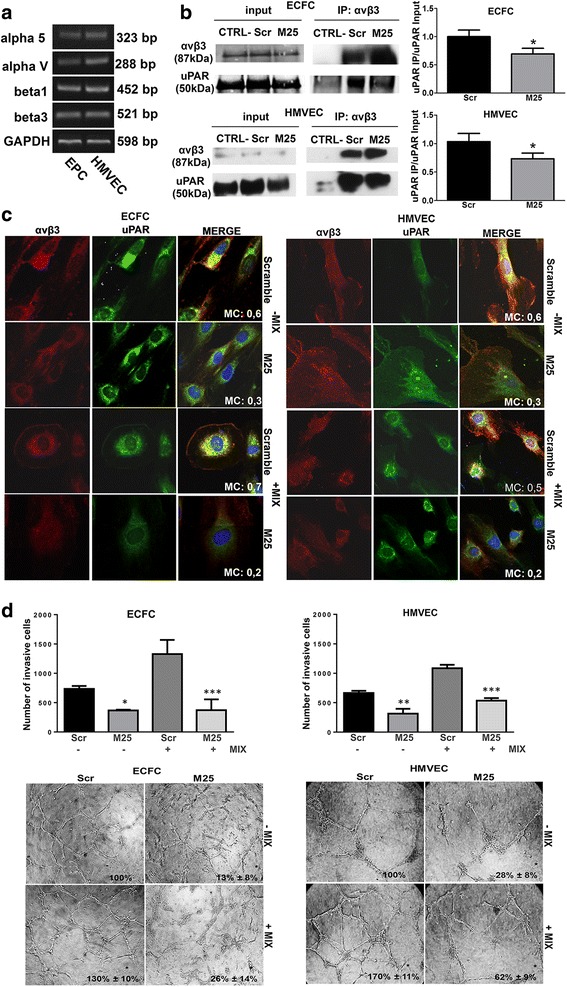
Integrin pattern and uPAR integrin interaction in amoeboid angiogenesis. a Semiquantitative PCR of the shown integrin α and β chains in ECFCs and HMVECs. GAPDH was used as a reference control. Product sizes, expressed in bp, are reported on the right. b Immunoprecipitation of αvβ3-integrin. Input: Western blotting of aliquots (30 μg of proteins) of cell lysates before immunoprecipitation, used as a reference loading control. IP αvβ3: immunoprecipitate (500 μg of proteins) obtained with anti-αvβ3-integrin antibody; alphav/beta3 lane: immunoblotting with anti-αvβ3 antibody; uPAR lane: immunoblotting with anti-uPAR antibody; CTRL-: a lysate that was treated with non-specific IgG (and Protein A/G) instead of the antibody and used as negative control. Molecular weights, expressed in kDa, are reported on the right. Histograms report band densitometry. Results are the mean of 3 different experiments performed in duplicate on each cell line and are shown as mean value ± SD. *: p < 0.05 significantly different from control c Confocal microscopy for αvβ3 integrin (red fluorescence) and uPAR (green fluorescence) co-localization in under mesenchymal (-MIX) and amoeboid (+MIX) conditions, in the absence and in the presence of M25 peptide and scramble M25 peptide (Scramble). Nuclear staining: DAPI (blue). The co-localization score is reported within each picture (MC: Manders’coefficient). Magnification: 40 X. The shown pictures are representative of 50 different pictures for each experimental condition and were studied by Image J analysis. d The upper panel shows Matrigel invasion under mesenchymal and amoeboid conditions untreated and treated with scramble M25 (Scr) and M25 peptide (M25), respectively. Histograms refer to quantification of Matrigel invasion assay obtained by counting the total number of migrated cells/filter. The lower panel shows in vitro angiogenesis at the same conditions described for Matrigel invasion. Numbers on the lower right side of each picture indicate the percent field occupancy of capillary plexus as described in the Materials and Methods section. Quantification was performed at 6 h after seeding and was obtained by scanning of six to nine photographic fields for each condition. Results are the mean of 5 different experiments performed in duplicate, on two different clones derived from two different donors, on each cell line and are shown as mean value ± SD. *: p < 0.05; **: p < 0,001; ***p < 0,0001 significantly different from control
