Highlights
-
•
HF increases pro-inflammatory cytokines levels in gastrocnemius.
-
•
Exercise training reverses the atrophy of gastrocnemius induced by HF.
-
•
Exercise training increases IL-10 and decreases TNF-α level in gastrocnemius.
Keywords: Skeletal muscle, Inflammation, Exercise training, Myocardial infarction, Rehabilitation
Abstract
Objective
This study examined the effects of exercise training (ExT) upon concentration of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) in the gastrocnemius of rats with heart failure (HF) induced by left coronary artery ligation.
Methods
Adult male Wistar rats submitted to myocardial infarction (MI) or sham surgery were randomly allocated into one of four experimental groups: trained HF (Tr-HF), sedentary HF (Sed-HF), trained sham (Tr-Sham) and sedentary sham (Sed-Sham). ExT protocol was performed on treadmill for a period of 8 weeks (60 m/days, 5×/week, 16 m/min), which started 6 weeks after MI. Cardiac hemodynamic evaluations of left ventricular end-diastolic pressure (LVEDP) and morphometric cardiac were used to characterize HF. The hemodynamic variables were recorded and gastrocnemius muscle was collected. TNF-α, IL-6 and IL-10 protein levels were determined by multiplex bead array.
Results
Sed-HF group presented increase of TNF-α level when compared with the Sed-Sham group (mean difference, MD 1.3; 95% confidence interval, CI −0.04 to 2.5). ExT reduced by 59% TNF-α level in Tr-HF group (MD −1.7; 95% CI −2.9 to −0.3) and increased IL-10 (MD 15; 95% CI 11–26) when compared with the Sed-HF group. Thus, the gastrocnemius muscle IL-10/TNF-α ratio was increased in Tr-HF rats (MD 15; 95% CI −8 to 47) when compared with the Sed-HF rats.
Conclusion
These results demonstrate that ExT not only attenuates TNF-α level but also improves the IL-10 cytokine level in skeletal muscle of HF rats.
Introduction
Heart failure (HF) is a complex and multifactorial syndrome associated with disability, morbidity and mortality.1, 2 Beyond neurohumoral activation, the inflammatory component plays an important role in the development of left ventricular dysfunction and peripheral myopathy, which in turn impair the functional capacity of patients with HF.3 Plasma TNF-α and interleukin-6 (IL-6) levels are correlated with severity of HF symptoms and oxygen consumption capacity upon exercise.4 The development of an anabolic-catabolic imbalance, with reduced anabolism and enhanced catabolism, is related to abnormalities in the neurohormonal systems and the activation of pro-inflammatory cytokines.5 Skeletal myopathy seems to be an important factor associated with exercise intolerance, fatigue, and dyspnea in HF patients.2
TNF-α may affect muscle metabolism and strength by stimulating expression of inducible nitric oxide synthase (iNOS) via nuclear-factor-kappa-B (NF-kB).6 Indeed, contractile dysfunction can result from TNF-α overexpression that signals via nitric oxide to decrease strength of skeletal muscle.7 Moreover, an increased expression of TNF-alpha and IL-6 was observed in skeletal muscle biopsies from patients with stable HF.8 As previously reported, seven-day subcutaneous administration of recombinant human IL-6 to rats resulted in a dose-dependent respiratory and peripheral skeletal muscle atrophy.9 Nevertheless, physical training decreases the expression of TNF-α in skeletal muscle that was accompanied by a reduction of atrophy in HF rats10 and humans.8
Exercise training (ExT) is associated with improvement of sympathetic and parasympathetic dysfunction in patients with HF.1 Recent studies have established a critical role of the sympathetic nervous system (SNS) in mediating interactions between the nervous and immune systems.11 Interleukin 10 (IL-10) could contribute to mediate the anti-inflammatory effects of ExT. Accumulating evidence suggests that ExT promotes anti-inflammatory benefits in HF experimental models12, 13 and clinical studies.14, 15 Although IL-10 is increased in plasma16 and soleus muscle13 in post-MI HF rats after treadmill endurance training, the effect of ExT on this cytokine in gastrocnemius muscle of HF rats is unclear.
Therefore, considering the important role of skeletal muscle in the release of cytokine, and the potential advantage of ExT in attenuating the loss of muscle mass and inflammation in chronic diseases, we evaluated the effects of ExT upon muscle mass, expression of TNF-α, IL-6 and IL-10 in the white gastrocnemius of rats with HF induced by left coronary artery ligation. In addition, we examined the balance between IL-10 and TNF-α production as an anti-inflammatory indicator following ExT.
Methods
Animals
Experiments were performed on 28 male Wistar rats weighing 220–270 g, obtained from the Animal Breeding Unit at the Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, RS, Brazil. The animals were allocated in groups of three to a cage with free access to water and pellet rodent chow diet and were maintained under standard conditions of temperature (22 °C). All procedures were performed in accordance with the Guideline for the Care and Use of Laboratory Animals (NIH Publication n° 85-23, revised 1996). All procedures outlined in this study were approved by the by the UFCSPA Ethics and Research Committee (protocol no. 39/11).
Experimental design
To induce myocardial infarction (MI), rats were anaesthetized with xylazine (12 mg/kg IP) and ketamine (90 mg/kg IP), intubated and artificially ventilated (SamWay VR 15) with a breathing rate of 60 breaths/min and oxygen inspired fraction of 100%. After thoracotomy, coronary artery ligation (CAL) was performed to induce MI and, subsequently HF.12 During the first 48 h, the animals were treated for post-operative pain with subcutaneous buprenorphine (0.15 mg/kg) and given a single dose of penicillin (20,000 U IP). After surgery, the rats remained six weeks in home cage for evolution of MI. This period is necessary to develop HF state.17 It was reported that in six weeks following MI the renal sympathetic nerve activity and left ventricular end-diastolic pressure (LVEDP) increase, and baroreflex control decreases,17 all of these changes are associated with chronic HF. Rats were randomly allocated into four experimental groups: sedentary sham-operated (Sed-Sham, n = 7), trained sham-operated (Tr-Sham, n = 7), sedentary HF (Sed-HF, n = 7) and trained HF rats (Tr-HF, n = 7).
Aerobic exercise training protocol
One week before the aerobic exercise training protocol the adaptation period was started to familiarize the rats with running on the treadmill with low speed (10 m/min) and short duration (10 min per session). Rats in the training groups (Sham and HF) performed an aerobic ExT program for 8 weeks (5×/week)16, 18 with speed at 16 m/min for 60 min/day.19 This intensity was maintained at a constant level throughout the experiment. Previous study demonstrated that this exercise protocol improved the aerobic capacity and citrate synthase activity in post-myocardial infarction rats.20 Only rats that ran steadily with little or no prompting were used in the study. The total duration of the experiment was 14 weeks (5 resting in home cage, 1 in adaptation to treadmill and 8 of aerobic ExT protocol).
Cardiac hemodynamic evaluation, infarct size, cardiac hypertrophy and pulmonary congestion
After a rest period of 48 h from the last training session, the cardiac hemodynamic evaluation was performed. The animals were anesthetized with xylazine (12 mg/kg IP) and ketamine (90 mg/kg IP), and a small incision in the anterior cervical region was performed for the insertion of a polyethylene catheter (PE-50) into the right carotid artery. The arterial pressure (AP) was recorded first during a 5-min period by connecting the arterial cannula to a pressure transducer (Miniature Pulse Transducer RP-155, Narco Biosystems, Houston, TX, USA), coupled to a pressure amplifier (General Purpose Amplifier 4, Model 2, Stemtech, Hudson, WI, USA). Then, the catheter was positioned inside the left ventricle (LV), and the pulse wave was monitored using the typical graphic registration of ventricular pressure and recorded for 5 min. Pressure analog signals were digitalized by a data acquisition system (AT/Codas, Dataq Instruments, Akron, OH, USA) at a sampling rate of 2000 Hz. These data were used to determine LV maximum change in pressure over time (+dP/dtmax) and LV minimum change in pressure over time (−dP/dtmax), and LVEDP.21 The last parameter was determined manually by detecting the point of inflection to the end of diastole via analysis of the ventricular pressure wave. The total left ventricle area and myocardial infarction scar were manually drawn on scanned images. ImageJ 1.47 software used planimetry to determine the size of the infarct. All photographs were analyzed independently by two blinded investigators. The heart weight-to-body weight ratio (HW/BW) and right ventricular to body weight ratio (RV/BW) values were determined. Lungs were dehydrated (80 °C) for 48 h and then weighed again to evaluate the water percentage. Cardiac hemodynamic evaluations of left ventricular diastolic function as well as morphometric cardiac were used to characterize HF. Only rats with elevated LVEDP ≥ 16 mmHg and myocardial infarction area ≥ 29% were included in the present study.16, 21
Skeletal muscle collection
After cardiac hemodynamic evaluation, the animals were euthanized with an overdose of anesthetic (thiopental 80 mg/kg IP), the superficial white gastrocnemius muscle were collected, weighed, immediately frozen in liquid nitrogen, and stored at −80 °C. The samples of fast-glycolytic muscles were obtained by dissection of gastrocnemius muscles, which were quickly excised from the left hindlimbs of the animals and dissected according to the muscle color (corresponding to myoglobin concentration) into a white portion derived from the superficial part of both lateral and medial head.
Gastrocnemius muscle sample preparation and determination of tissue TNF-α, IL-6 and IL-10 levels
The gastrocnemius muscles were chosen for cytokine assays because of their metabolic characteristics and tend to be more affected in disease conditions.10 The gastrocnemius samples were homogenized in potassium phosphate buffer (KPi, pH 7.4) containing 4.08 g/L KH2PO4, 8.9 g/L KCl, 8.71 μg/mL phenylmethylsulfonyl fluoride (PSMF), 0.1 μg/mL aprotinin, 0.1 μg/mL leupeptin and 0.1 μg/mL pepstatin proteases inhibitors using a hand-held homogenizer. The homogenates were centrifuged at 12,000 × g (Mikro 220 R, Hettich Zentrifugen, Tuttlingen, Germany) for 60 min at 4 °C. The supernatant was removed and TNF-α, IL-6 and IL-10 levels were determined by multiplex bead array using Milliplex™ MAP rat cytokine kits (RCYTO-80K) (Millipore, Billerica, MA, USA). All samples were run in duplicates and the average value is reported as pg/mL.
Statistical analyses
The data are presented as the mean ± SD. The data were tested for normal distribution using the Kolmogorov–Smirnov test. Comparisons were made between groups using two-way ANOVA (exercise training and HF as the main factors) followed by the Newman–Keuls post hoc test. When Gaussian normality failed, a Kruskal–Wallis test on ranks and Dunn's test were performed. Unpaired student t test compared infarcted areas between the Sed-HF and Tr-HF groups. Between-group differences are reported as the mean difference (MD) and 95% confidence interval (95% CI). A p value < 0.05 was considered statistically significant.
Results
Infarcted area, cardiac hypertrophy, pulmonary congestion, body and muscle weights
The perioperative mortality in the HF-groups was 35%. The infarct sizes of the LV were similar in two infarcted groups, (MD −3.1; 95% CI −9 to 3; p = 0.4). At the end of study, between-group analyses showed that HW/BW and RV/BW ratio, and pulmonary congestion were higher in the Sed-HF group when compared with Sed-Sham group (p < 0.01). ExT reduced pulmonary congestion (p = 0.03 for group), cardiac hypertrophy (HW/BW, p = 0.03 for interaction effect) and RV hypertrophy (RV/BW, p = 0.01 for interaction effect) when compared with Sed-HF group, respectively. After 14 weeks post-MI, the Sed-HF group showed a significant reduction between-group analyses in the muscle weight and ratio of muscle weight to body weight of the gastrocnemius muscle when compared with Sed-Sham group (p = 0.01), suggesting muscle atrophy. However, when the exercise-trained HF group was compared with the Sed-HF group, there was an increase in the weight (p = 0.02) and ratio of weight to body weight of the gastrocnemius muscle (p = 0.01). There was no significant difference in the body weight among all the groups. All of these data are summarized in Table 1.
Table 1.
Body and muscle weights, morphometric cardiac characteristics, and pulmonary congestion in sham and HF rats after 8 weeks of exercise training.
| Groups | Sedentary | Trained | MD [95% CI] |
|---|---|---|---|
| BW, g | |||
| Sham | 347 (20) | 353 (24) | 6 [−22 to 34] |
| HF | 338 (28) | 352 (29) | 14 [−23 to 51] |
| MD [95% CI] | −8.8 [−53 to 36] | −0.8 [−29 to 28] | |
| HW/BW, mg/g | |||
| Sham | 2.69 (0.1) | 2.7 (0.1) | −0.01 [−0.1 to 0.1] |
| HF | 3.9 (0.1)* | 3.43 (0.4)† | −04 [−0.9 to 0.02] |
| MD [95% CI] | 1.2 [1 to 1.3] | 0.7 [0.2 to 1.2] | |
| RV/BW, mg/g | |||
| Sham | 0.56 (0.06) | 0.57 (0.15) | 0.01 [−0.1 to 0.1] |
| HF | 1.39 (0.2)* | 0.93 (0.37)† | −0.4 [−0.9 to −0.02] |
| MD [95% CI] | 0.8 [0.6 to 1] | 0.3 [−0.05 to 0.7] | |
| Pulmonary congestion, % | |||
| Sham | 73.6 (4.6) | 74.1 (1) | 0.45 [−3.9 to 4.8] |
| HF | 79.9 (0.9)* | 76.7 (1.2)† | −3.1 [−4.2 to −2.1] |
| MD [95% CI] | 6.3 [1.7 to 10] | 2.7 [1.5 to 3.9] | |
| Gastrocnemius, g | |||
| Sham | 1.92 (0.16) | 1.99 (0.22) | 0.07 [−0.1 to 0.3] |
| HF | 1.62 (0.18)* | 1.91 (0.26)† | 0.2 [−0.01 to 0.5] |
| MD [95% CI] | −0.3 [0.5 to −0.04] | −0.08 [−0.3 to 0.2] | |
| Gastrocnemius/BW, mg/g | |||
| Sham | 5.54 (0.4) | 5.63 (0.3) | 0.08 [−0.4 to 0.5] |
| HF | 4.8 (0.5)* | 5.4 (0.3)† | 0.5 [−0.07 to 1.2] |
| MD [95% CI] | −0.7 [−1.2 to −0.2] | −0.2 [−0.6 to 0.2] | |
Values are means (standard derivation); n = 7 for all groups. HF, heart failure group; BW, body weight; HW/BW, heart weight to BW ratio; RV/BW, right ventricle to BW ratio; MD, mean difference; CI, confidence interval.
p < 0.05 compared with Sed-Sham and Tr-Sham.
p < 0.05 compared with Sed-HF.
Hemodynamic data
Table 2 shows the hemodynamic characteristics in sham and HF rats. As expected, LVEDP was elevated in both HF groups compared with the sham groups (p < 0.001 for group effect). However, when the trained HF group was compared with the Sed-HF group, there was an improvement in diastolic function (p = 0.01 for training effect). This result suggests that 8-week ExT have a beneficial hemodynamic effect in HF rats. The negative derivative of LV pressure (−dP/dtmax) was significantly reduced in the HF groups compared with the sham groups (p < 0.05 for group effect). No differences were observed in mean arterial pressure (MAP) or positive derivative of LV pressure (+dP/dtmax). All hemodynamic variables were assessed while the rats were under anesthesia.
Table 2.
Hemodynamic characteristics in sham and HF rats after 8 weeks of exercise training.
| Groups | Sedentary | Trained | MD [95% CI] |
|---|---|---|---|
| MAP, mmHg | |||
| Sham | 102.5 (9) | 98.6 (11) | −3.8 [−15 to 7.5] |
| HF | 96.6 (15) | 95.6 (10.3) | −0.4 [−11 to 10] |
| MD [95% CI] | −6.3 [−21 to 8.2] | −2.9 [−19 to 13] | |
| LVEDP, mmHg | |||
| Sham | 5.32 (1.5) | 4.1 (1.1) | −1.2 [−2.7 to 0.3] |
| HF | 22.6 (5.3)* | 18.1 (1.8)*,† | −4.4 [−9 to 0.1] |
| MD [95% CI] | 17 [11 to 23] | 14 [11 to 16] | |
| +dP/dtmax, mmHg/s | |||
| Sham | 5.537 (1.673) | 5.979 (1.006) | 442 [−1723 to 2607] |
| HF | 5.271 (1.450) | 5.192 (956) | −79 [−1012 to 854] |
| MD [95% CI] | 266 [−2029 to 1496] | −787 [−2398 to 822] | |
| −dP/dtmax, mmHg/s | |||
| Sham | −4.351 (1.039) | −4.553 (1.173) | −202 [−1933 to 1529] |
| HF | −3.446 (710)* | −3.407 (379)* | 39 [−528 to 607] |
| MD [95% CI] | 904 [−139 to 1949] | 1146 [−104 to 2397] | |
Values are means (standard derivation); n = 7 for all groups. HF, heart failure group; MD, mean difference; CI, confidence interval; MAP, mean arterial pressure; LVEDP, LV end-diastolic pressure; +dP/dtmax, maximum positive LV derivate; −dP/dtmax, maximum negative LV derivate.
p < 0.05 compared with Sed-Sham and Tr-Sham.
p < 0.05 compared with Sed-HF.
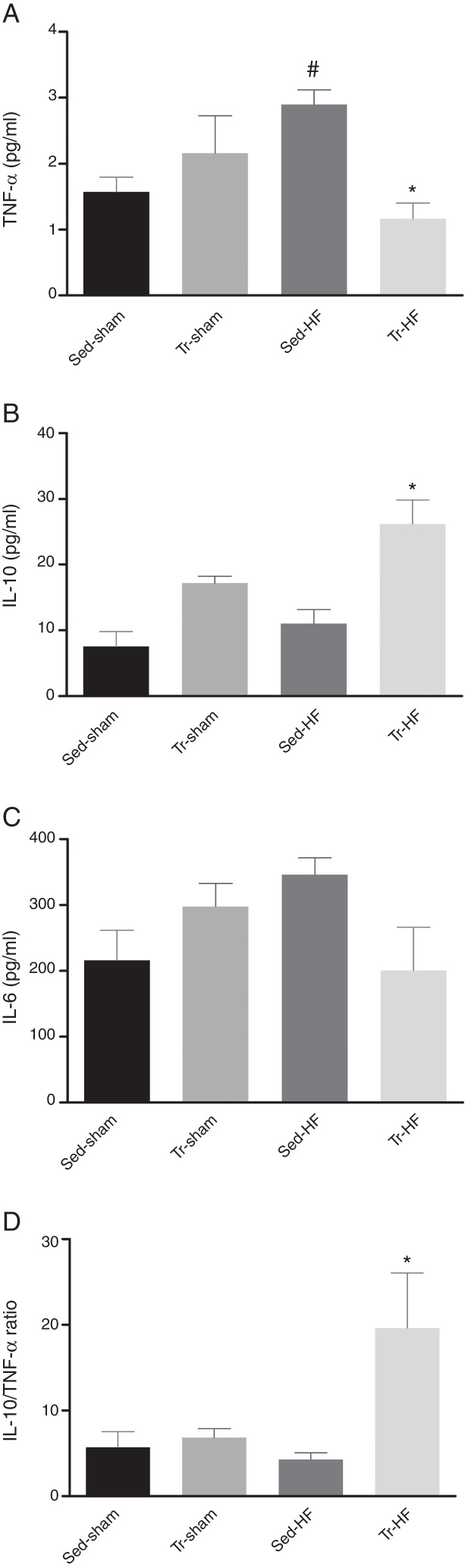
Gastrocnemius muscle TNF-α, IL-6 and IL-10 levels
Muscular levels of TNF-α (Fig. 1A) were elevated in the Sed-HF group when compared with the Sed-Sham group (MD 1.3; 95% CI −0.04 to 2.5; p < 0.05 for group effect). ExT significantly reduced by 59% TNF-α level in Tr-HF group when compared with the Sed-HF group (MD −1.7; 95% CI −2.9 to −0.3; p = 0.02 for interaction effect). We observed an increase in IL-10 (Fig. 1B) in trained HF group when compared with the Sed-HF (MD 15; 95% CI 11–26; p = 0.01) and Tr-Sham group (MD 9; 95% CI −6 to 27; p = 0.01 for group and training effects), respectively. However, no differences were found in the IL-6 (Fig. 1C) among groups. The gastrocnemius muscle IL-10/TNF-α ratio (Fig. 1D) was significantly higher in the Tr-HF group than in the Sed-HF and Sham groups (p < 0.01 for training and interaction effects). Cytokine analyses were performed only with muscle samples feasible.
Figure 1.
Mean data showing the effects of exercise training in the gastrocnemius muscle of anti- and pro-inflammatory cytokines. (A) Tumor necrosis factor-alpha (TNF-α); * p < 0.05 vs. Sed-HF; # p < 0.05 vs. Sed-Sham. (B) Interleukin-10 (IL-10); * p < 0.05 vs. Sed-Sham and Sed-HF. (C) interleukin-6 (IL-6). (D) IL-10/TNF-α ratio. * p < 0.01 vs. all groups. Values are the means ± SD, (n = 3–6 per groups).
Discussion
The main findings of this study were that exercise training was able to reverse the muscle atrophy and improve inflammatory profile in skeletal muscle of HF rats. Evidence of this effect was provided by reduction of TNF-α level, as well as by the increase of IL-10 level and IL-10/TNF-α ratio in gastrocnemius muscle. In addition, the beneficial effect of ExT was accompanied by the decrease in LVEDP.
Chronic HF is associated with wasting muscle and intolerance to exercise induced by elevated pro-inflammatory cytokines, neurohumoral alterations, and reactive oxygen species (ROS) production.8, 22, 23 Among skeletal muscle abnormalities, elevated TNF-α can modulate immune and inflammatory response, exacerbating muscle wasting, which was found upregulated in gastrocnemius muscle of HF rats. The result of HF-induced muscle atrophy matched other studies.10, 24 It was suggested that elevated TNF-α level could stimulate expression of the myostatin via NF-kB.25 The present work also demonstrates that after 8 weeks of ExT, there was a restoration in TNF-α level and increase of gastrocnemius mass in HF rats. Our results support previous studies in humans8 and animal models10 suggesting that ExT can be used as an effective intervention to prevent skeletal muscle wasting.22, 26
The present study extends previous reports developed from our laboratory concerning the anti-inflammatory effects of aerobic12, 16 and resistance21 training in HF rats. Regarding skeletal muscle IL-10, the current study demonstrated that 8-week ExT program after MI increased IL-10 in gastrocnemius. A study reported similar improvement in soleus IL-10 levels by physical exercise in HF rats.13 Likewise, ExT-induces IL-10 expression in paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM) of spontaneously hypertensive rats.27 Furthermore, in healthy rats the ExT increased IL-10 in white adipose tissue of mesenteric depot.28 All these studies suggest that ExT improves the anti-inflammatory defense mechanism by the increase of IL-10 at different tissues. A network of molecular and metabolic pathways could mediate this adaptation. Muscle contraction, which triggers intracellular signaling cascades, could activate such a network. During exercise, the contraction of skeletal muscle produces IL-6 and stimulates anti-inflammatory cytokines, such as IL-1 receptor antagonist and IL-10, and inhibits the liberation of pro-inflammatory cytokine TNF-α.14, 29 At the molecular level, IL-10's anti-inflammatory effects are mediated through indirect actions of the signal transducer and activator of transcription 3 (STAT3) on target inflammatory genes.30 STAT3-induced transcriptional inhibitor can selectively control transcription at inflammatory promoters, like TNF-α.30
Although IL-6 has been most studied in the healthy skeletal muscle,29 its role in HF remains unclear, regarding both acute and chronic physical exercise responses.15 Studies from our laboratory have shown that aerobic exercise and resistance training can reduce plasma IL-6 level in HF rats.16, 21 The results presented here display decreasing trends in gastrocnemius IL-6 level in HF. Similarly, Gielen et al.8 has shown that ExT may lower intramuscular IL-6 in the absence of serum changes in patients with HF. It remains to be determined if the training adaptations occur in plasma or locally within the skeletal muscle. Furthermore, it was suggested that the major role of IL-6 is to regulate the metabolism rather than acting as an inflammatory mediator.31
We observed loss in gastrocnemius muscle mass. Animal model of HF32 has demonstrated that faster-twitch, more glycolytic fibers lose more vascularization and enhanced E3 ligase MAFBx/Atrogin gene expression. In addition, upregulation of autophagy-related genes appeared only in plantaris muscle of infarcted rats.24 However, the cross-sectional area of the gastrocnemius muscle was not changed after 6 months post-induced myocardial infarction33 but reduction in the type I fibers in soleus was reported by others.10 It is possible that muscle fiber type-specific can be differentially affected during developed of HF.
Besides the anti-inflammatory benefits, regular exercise training seems able to induce hemodynamic adaptations in HF rats.12, 16, 26, 34 Our results showed a significant reduction in LVEDP, HW/BW and RV/BW after ExT in HF rats, indicating positive effect on cardiopulmonary function. This finding is in line with previous reports showing that low to moderate treadmill running offers beneficial effects on the ventricles of HF rats.16 Several mechanisms have been related to hemodynamic improvement such as enhanced antioxidant enzyme capacity, restoration of β-adrenergic signaling and attenuation of oxidative stress in myocardium.20, 26
In conclusion, this study demonstrates that ExT benefits inflammatory modulation, especially by increase of IL-10 level in gastrocnemius muscle. In addition, ExT attenuates TNF-α level as well as avoids gastrocnemius atrophies. The improvement in the balance of pro- and anti-inflammatory cytokine induced by ExT prevents skeletal muscle inflammation and reduces muscle wasting in HF state.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to acknowledge the colleagues from Grupo de Pesquisa em Interação Cardiopulmonar (GPIC) of Universidade Federal de Ciências da Saúde de Porto Alegre (Brazil) for their support and assistance.
References
- 1.Piepoli M.F. Exercise training in chronic heart failure: mechanisms and therapies. Neth Heart J. 2013;21(February (2)):85–90. doi: 10.1007/s12471-012-0367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgiadou P., Adamopoulos S. Skeletal muscle abnormalities in chronic heart failure. Curr Heart Fail Rep. 2012;9(June (2)):128–132. doi: 10.1007/s11897-012-0090-z. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs M., Drexler H. Chronic heart failure and proinflammatory cytokines: possible role of physical exercise. Exerc Immunol Rev. 2004;10:56–65. [PubMed] [Google Scholar]
- 4.Torre-Amione G., Kapadia S., Benedict C., Oral H., Young J.B., Mann D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27(April (5)):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 5.Anker S.D., von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90(April (4)):464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y.P., Schwartz R.J., Waddell I.D., Holloway B.R., Reid M.B. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12(July (10)):871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 7.Stasko S.A., Hardin B.J., Smith J.D., Moylan J.S., Reid M.B. TNF signals via neuronal-type nitric oxide synthase and reactive oxygen species to depress specific force of skeletal muscle. J Appl Physiol (1985) 2013;114(June (11)):1629–1636. doi: 10.1152/japplphysiol.00871.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gielen S., Adams V., Möbius-Winkler S. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(September (5)):861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 9.Janssen S.P., Gayan-Ramirez G., Van den Bergh A. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111(March (8)):996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 10.Souza R.W., Piedade W.P., Soares L.C. Aerobic exercise training prevents heart failure-induced skeletal muscle atrophy by anti-catabolic, but not anabolic actions. PLoS ONE. 2014;9(10):e110020. doi: 10.1371/journal.pone.0110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(July (3)):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes R.B., Tonetto M., Machado N. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol (1985) 2008;104(June (6)):1641–1647. doi: 10.1152/japplphysiol.00062.2008. [DOI] [PubMed] [Google Scholar]
- 13.Batista M.L., Rosa J.C., Lopes R.D. Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine. 2010;49(January (1)):102–108. doi: 10.1016/j.cyto.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98(April (4)):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 15.Smart N.A., Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail. 2011;17(May–June (3)):110–114. doi: 10.1111/j.1751-7133.2011.00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Nunes R.B., Alves J.P., Kessler L.P., Dal Lago P. Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clinics (Sao Paulo) 2013;68(June (6)):876–882. doi: 10.6061/clinics/2013(06)24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis J., Weiss R.M., Wei S.G., Johnson A.K., Felder R.B. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281(November (5)):R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- 18.Xu X., Wan W., Powers A.S. Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. J Mol Cell Cardiol. 2008;44(January (1)):114–122. doi: 10.1016/j.yjmcc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Véras-Silva A.S., Mattos K.C., Gava N.S., Brum P.C., Negrão C.E., Krieger E.M. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol. 1997;273(December (6 Pt 2)):H2627–H2631. doi: 10.1152/ajpheart.1997.273.6.H2627. [DOI] [PubMed] [Google Scholar]
- 20.Wan W., Powers A.S., Li J., Ji L., Erikson J.M., Zhang J.Q. Effect of post-myocardial infarction exercise training on the renin-angiotensin-aldosterone system and cardiac function. Am J Med Sci. 2007;334(October (4)):265–273. doi: 10.1097/MAJ.0b013e318068b5ed. [DOI] [PubMed] [Google Scholar]
- 21.Alves J.P., Nunes R.B., Stefani G.P., Dal Lago P. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure. PLoS ONE. 2014;9(10):e110317. doi: 10.1371/journal.pone.0110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen T.S., Schuler G., Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6(September (3)):197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zizola C., Schulze P.C. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev. 2013;18(September (5)):623–630. doi: 10.1007/s10741-012-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jannig P.R., Moreira J.B., Bechara L.R. Autophagy signaling in skeletal muscle of infarcted rats. PLoS ONE. 2014;9(1):e85820. doi: 10.1371/journal.pone.0085820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenk K., Schur R., Linke A. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11(April (4)):342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 26.Adams V., Niebauer J. Reversing heart failure-associated pathophysiology with exercise: what actually improves and by how much? Heart Fail Clin. 2015;11(January (1)):17–28. doi: 10.1016/j.hfc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal D., Welsch M.A., Keller J.N., Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol. 2011;106(November (6)):1069–1085. doi: 10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lira F.S., Rosa J.C., Yamashita A.S., Koyama C.H., Batista M.L., Seelaender M. Endurance training induces depot-specific changes in IL-10/TNF-alpha ratio in rat adipose tissue. Cytokine. 2009;45(February (2)):80–85. doi: 10.1016/j.cyto.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Brandt C., Pedersen B.K. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray P.J. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102(June (24)):8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(October (4)):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 32.Li P., Waters R.E., Redfern S.I. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol. 2007;170(February (2)):599–608. doi: 10.2353/ajpath.2007.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lima A.R., Martinez P.F., Okoshi K. Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure. Int J Exp Pathol. 2010;91(February (1)):54–62. doi: 10.1111/j.1365-2613.2009.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes R.B., Alves J.P., Kessler L.P., Dornelles A.Z., Stefani G.P., Lago P.D. Interval and continuous exercise enhances aerobic capacity and hemodynamic function in CHF rats. Braz J Phys Ther. 2015;19(July–August (4)):257–263. doi: 10.1590/bjpt-rbf.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]