Abstract
Background
We examined the effect of antioxidant supplementation and exercise on irisin within postmenopausal women.
Methods
Forty-eight participants (age: 55.7 ± 4.9 years; weight: 68.0 ± 6.3 kg; BMI 27.0 ± 2.7; mean ± SD) were randomized into four groups for the eight week intervention: control group (CG; n = 12), resistance training group (RTG; n = 12), supplementation with Zataria multiflora group (ZG; n = 12), or supplementation with Z. multiflora and resistance training group (ZRTG; n = 12). RTG and ZRTG performed circuit resistance training, and both ZG and ZRTG consumed 500 mg of Z. multiflora every day during the intervention. Blood samples were taken 48 hours before and after the intervention.
Results
There was a significant difference in irisin at post-training, with greater levels in ZRTG compared to CG. A significant increase was noted for irisin at post-training compared to pre-training for ZG, RTG, and ZRTG. Moreover, we identified a significant decrease in malondialdehyde in the RTG and ZRTG groups and increase in glutathione in the ZG, RTG, and ZRTG groups when compared to CG.
Conclusion
These findings showed that exercise, Z. multiflora supplementation or their combination led to an increase in irisin.
Keywords: Antioxidants, Circuit resistance training, Myokine, Postmenopausal women, Zataria multiflora
1. Introduction
In women, menopause-induced changes in hormones contribute to muscular atrophy, accelerated bone loss, and elevated oxidative stress, and consequently menopause may increase the physiological decline associated with aging and inactivity.1, 2, 3
Engagement in physical activity plays an important role in opposing physiological decline associated with aging and menopause.4 A potential mechanism by which exercise may slow the effects of aging is by altering antioxidant status, an important component pathway in tissue damage and repair. Previously an acute exercise bout has been shown to increase oxygen consumption resulting in elevated generation of reactive oxygen species (ROS) in skeletal muscle.5 Chronic exercise training on the other hand, may reduce inflammatory oxidative damage by increasing antioxidant capacity and reducing oxidative stress.6
In many cultures, certain plants are used as traditional medicines and ascribed anti-aging properties. Recently, there is a growing body of literature investigating the potential beneficial roles of many of these plants on physiological function including antioxidant capacity.7 One such plant, which has important applications in traditional Iranian medicine, is Zataria multiflora, a plant belonging to the Lamiaceae family which contains various antioxidative components such as Thymol and Carvacrol.7 Supplementation with Zataria extract has been shown to exhibit concentration-dependent radical-scavenging activity on 1,1-diphenyl-2-picryl-hydrazyl free radicals.8
Numerous exercise-induced myokines have been identified and recently, a newly discovered myokine, irisin has attracted significant attention.9 Irisin is a peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α)-dependent and exercise-responsive myokine that stimulates browning of white adipocytes10 and potentially has a role in antioxidant defense,11, 12, 13 Boström et al. (2012) described expression of the irisin gene in skeletal muscle,14 and aerobic exercise training has previously been reported to increase plasma irisin concentrations in post-menopausal women.15
To the best of our knowledge, the effects of plants with antioxidative properties such as Z. multiflora in combination with exercise training on the expression and activity of skeletal muscle myokines with roles in antioxidant status have not been investigated in postmenopausal women. Consequently, the current study examined the combined effect of Z. multiflora extract and circuit resistance exercise training (CRT) on the novel myokine irisin and antioxidant status (malondialdehyde, glutathione and total antioxidant capacity) in a group of post-menopausal women.
2. Methods
2.1. Study design
Following baseline testing, participants were matched based on weight, height, and body mass index (BMI), and then randomly divided into four equal groups by a person independent of the trial with group allocation provided in sequentially numbered opaque sealed envelopes. The four groups consisted of a control group (CG), who received eight weeks of usual care, a resistance training group (RTG), who received an eight week supervised circuit resistance training program, a Z. multiflora group (ZG) who received daily supplementation with a Z. multiflora supplement for eight weeks, and a Z. multiflora and resistance training group (ZRTG) who received both the eight-week circuit resistance training program and daily Z. multiflora supplementation. A number of studies have previously demonstrated resistance training to be a safe and effective exercise modality in post-menopausal women.16, 17, 18 Ethics approval was obtained through the local Education Ethical Research Committee (Number: 4115), and the study was conducted in accordance with the Declaration of Helsinki.
2.2. Participants
Women were eligible for inclusion if they were at least six months’ post-menopause (as confirmed by a gynecologist), had no addiction to drugs or alcohol, had no recent participation (last 6 months) in a planned exercise program, no history of renal, hepatic, cardiovascular disease, diabetes, and/or any physical injury or problem preventing participation in an exercise program. Postmenopausal status was confirmed by postmenopausal levels of serum estradiol (<120 pmol/L) and follicle-stimulating hormone (FSH > 30 IU/L).19, 20 Participants were advised that no new exercise should be commenced and not to use non-prescription medications and supplements during the trial. Before participating in the study, all procedures were explained to volunteers and after complete awareness of the study terms, and the completion of a medical questionnaire, written informed consent was obtained.
2.3. Zataria essential oil preparation and supplementation
Z. multiflora leaves were collected in March around the gardens of Eghlid, Shiraz. Then, they were dried in the shade for 10 days. Following drying in the shade the Z. multiflora leaves were dried in an oven for 48 hours at a temperature of 32°C and then powdered using a Chinese pounder. Fifty grams of the sample powder was extracted for 3 hours using water distillation with a Clevenger apparatus at 100°C. The extract was filtered, then dried on anhydrous sodium sulfate, and finally transferred into a sealed glass container and stored at 4°C. The extract yield was calculated as dried oil volume divided by the initial dry powder weight multiplied by 100. The calculated yield was 3%.
2.4. Zataria essential oil combination by gas chromatography–mass spectrometry (GC–MS)
GC–MS was used to isolate and identify the components of Z. multiflora essential oil. GC–MS analysis was performed using Agilent 5975 mass spectrometer detector (MSD) coupled with gas chromatography model of Agilent USA GC 7890A MS 5975C. A column of welded silica HP-5 (5% phenyl, 1.95% polydimethyl siloxane) with profile of 30 × 0.25 mm2 and film thickness of 0.25 μm was used. Helium was used as the carrying gas and the flow rate of gradient was 1 mL/min. The thermal program used was as follows: first column temperature was set at 50 °C for 5 minutes, then increased at 3°C/minute to 240°C, then increased at 5°C/minute to 300°C, and finally set for 3 minutes at this temperature. Essential oil samples were diluted by n-hexane at a ratio of 1:10 and 1 μL of the resultant solution was then injected into the gas chromatograph. The temperature at the injector and detector was fixed at 290°C. Compounds in Z. multiflora essential oil were identified using the fragmentation pattern in the database of wiley7n.land NIST08 and also using retention time in the chromatography column. For each combination, the ratio of the level below peak was determined to the total levels below peak of all compounds and the results are summarized in Table 1.
Table 1.
Compounds of Zataria multiflora
| Compounds | Inhibition time (min) | Under peak level (%) |
|---|---|---|
| Thymol | 35.90 | 26.8 |
| Carvacrol | 36.50 | 22.9 |
| p-Cymene | 21.73 | 7.7 |
| γ-Terpinene | 23.55 | 6.8 |
| α-Pinene | 16.53 | 3.2 |
| β-Caryophyllene | 41.36 | 3 |
| Carvacrol methyl ether | 32.86 | 2.4 |
| α-Terpinene | 21.12 | 2.2 |
| Spathulenol | 47.96 | 2 |
| Linalool | 25.59 | 1.8 |
| β-Myrcene | 19.60 | 1.5 |
| Total | – | 80.3 |
For supplementation, 500 mg of dry Z. multiflora leaves and powder were cast in capsules using a Chinese oven. Both ZG and ZRTG consumed 500 mg of Z. multiflora every day of the eight-week intervention. This consisted of one capsule (500 mg) with 100 mL of water after breakfast. RTG and CG consumed placebo capsules (500 mg wheat flour) with 100 mL of water after breakfast.
2.5. Resistance training
Following the familiarization week which involved training participants in correct lifting techniques, one repetition maximum (1RM) of the prescribed movements was determined using the Brzycki equation.21 Training sessions were delivered using resistance circuit format with alternation between upper-body and lower-body movements as well as multi-joint movements. The exercises included: 1, squat; 2, chest press; 3, leg press; 4, standing military press; 5, knee extension; 6, seated cable rowing; 7, knee curl; 8, biceps curl, 9, standing calf raise; 10, triceps press; 11, back extension, and 12, abdominal crunch. The participants in both exercise training groups performed the movements at 55% of 1RM for eight weeks (3 sessions/week). Each exercise session included a 5 minutes warm-up and then followed with the 12 exercises. Each exercise was performed for a duration of 30 seconds, following which participants moved smoothly to the next exercise without a rest. The number of repetitions at each station was recorded for the participants. In each session, two sets of the 12 exercises were carried out with a 3 minutes active rest between each set.22
2.6. Blood sampling
Participants were requested to comply with the following conditions prior to each blood sample: 1) avoid use of non-prescription medications and supplements, 2) avoid any strenuous exercise other than the exercise prescribed as part of the study for at least 72 hours before the test, 3) to avoid coffee, dark tea, bananas, cereal and heavy or greasy foods at least 24 hours before the test, and 4) to match their diet in the 48 hours prior to each blood sample. Blood samples were taken following a 12-hour overnight fast a minimum of 48 hours before the first exercise session and 48 hours after the last exercise training session.
2.7. Dietary analysis
Participants were requested to complete a two-day food diary prior to both the baseline and endpoint blood sampling. Previously, a single-day of dietary analysis has been considered sufficient to assess compliance to dietary advice.23 The amount of nutrients consumed was calculated using previously described methods.23, 24
2.8. Irisin measurement
Human serum irisin levels were measured by sandwich ELISA as per manufacturer's instructions (CUSABIO, product number: CSB-EQ027943HU). The assay has high sensitivity (minimal detection dose less than 0.78 ng/mL) and specificity with no significant cross-reactivity between human irisin and analogs. Briefly, serum samples were left to coagulate at room temperature and centrifuged at 3000 × g for 15 minutes. One hundred microliters of sample and standards were loaded per well and the optical density was determined at 450 nm, and corrected for optical plate imperfections at 540 nm.
2.9. Antioxidant measurement
Malondialdehyde (MDA) was measured using the thiobarbituric acid method as previously described25 with spectrophotometer (Apel, Japan). Total glutathione (GSH) was measured utilizing an enzymatic recycling method as per manufacturer's instructions (Cayman Chemical Company, USA, Catalog number 703002). Briefly, serum samples were deproteinated, and GSH was measured by oxidation to produce 5-thio-2-nitrobenzoic acid (TNB) which was read colorimetrically at 405–414 nm on a plate reader (Biohit, Finland).
Total antioxidant capacity (TAC) was determined through ferric reducing/antioxidant power (FRAP) as previously described.26 Briefly, antioxidants present in serum reduce the Fe3+/tripyridyltriazine complex to blue colored ferrous form with an increase in absorbance at 593 nm. The change in absorbance is proportional to the combined FRAP value of the antioxidants in the sample.
2.10. Statistical analysis
All data were analyzed using software SPSS Version 20. The normality of data was approved by Kolmogorov–Smirnov test. Repeated measures analysis of variance (ANOVA) (4 × 2) was used to compare data of the four groups with post hoc analyses conducted using the Bonferroni method to locate means that were significantly different. Statistical significance was considered at the level of p < 0.05.
3. Results
Forty-eight participants (age: 55.7 ± 4.9 years; weight: 68.0 ± 6.3 kg; BMI 27.0 ± 2.7; mean ± SD) who met the inclusion criteria, consented to participate and underwent baseline testing (Fig. 1). Participants were randomly allocated to each of the four intervention groups: CG (n = 12), RTG (n = 12), ZG (n = 12), ZRTG (n = 12) and all participants completed the assigned intervention. Demographic characteristics of the participants at baseline and endpoint are provided in Table 2. No significant differences were noted in any demographic parameter between groups at baseline. No significant changes were noted for any demographic parameters in the CG. Fat mass significantly decreased at post-intervention in the ZG. Body weight, BMI, and fat mass significantly decreased at post-intervention while muscle mass increased in the RTG and ZRTG. Significant differences, however, were noted between groups at endpoint, with lower values for BMI in the ZG and RTG, and lower values for fat mass in the ZG, RTG, and ZRTG, when compared to the CG. Further, muscle mass was significantly higher in the ZRTG when compared to the ZG. Further, there were no differences within or between treatment groups in dietary intake in the two days prior to baseline or endpoint blood sampling (Table 3).
Fig. 1.
CONSORT flow chart.
Table 2.
Demographic Characteristics of Participants at Baseline and Endpoint (Mean ± SD).
| Groups | CG |
ZG |
RTG |
ZRTG |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | Baseline | Endpoint | Baseline | Endpoint | |
| Age (years) | 56.5 ± 4.2 | – | 54.4 ± 3.9 | – | 58.03 ± 4.7 | – | 53.8 ± 6.0 | – |
| Height (cm) | 156.7 ± 3.0 | – | 160.9 ± 4.0 | – | 158.7 ± 3.8 | – | 159.2 ± 4.6 | – |
| Weight (kg) | 68.7 ± 13.3 | 68.7 ± 4.7 | 66.4 ± 10.9 | 65.9 ± 5.1 | 67.1 ± 7.2 | 64.3 ± 5.6* | 69.9 ± 5.7 | 67 ± 4* |
| BMI (kg/m2) | 27.9 ± 2.2 | 27.9 ± 1.8 | 25.6 ± 2.2 | 25.4 ± 1.6# | 26.6 ± 3.1 | 25.5 ± 2.3*# | 27.6 ± 2.7 | 26.4 ± 2.1* |
| Fat mass (%) | 20.6 ± 1.7 | 20.5 ± 1.6 | 19.9 ± 1.9 | 18.6 ± 1.4*#† | 20.1 ± 2.1 | 17.1 ± 1.9*# | 21 ± 1.4 | 18 ± 1.1*# |
| Muscle mass (kg) | 27.6 ± 2.5 | 27.6 ± 2.3 | 26.5 ± 2.5 | 26.8 ± 2.1 | 26.8 ± 2.8 | 28.4 ± 2.2* | 28 ± 1.9 | 29.1.9* |
Data are expressed as mean ± SD. BMI – body mass index, CG – control group, ZG – Zataria group, RTG – resistance training group, ZRTG – Zataria and resistance training group; *p < 0.05 vs. baseline in same group; #p < 0.05 vs. CG at endpoint, †p < 0.05 vs. ZRTG at endpoint.
Table 3.
Food Intakes (Mean ± SD) of Study Groups for 2 Days Before the Pretest and Posttest Blood Sampling.
| Groups | CG |
ZG |
RTG |
ZRTG |
||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Total calorie (kcal/d) | 2225 ± 190 | 2396 ± 103 | 2203 ± 157 | 2317 ± 98 | 2142 ± 125 | 2276 ± 180 | 2096 ± 113 | 2162 ± 125 |
| Total protein (g/d) | 111 ± 16 | 112 ± 17 | 112 ± 14 | 110 ± 18 | 110.5 ± 17 | 108 ± 19 | 104 ± 16 | 102 ± 19 |
| Protein (g/kgBW/d) | 1.05 ± 0.45 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Total protein (% energy) | 3.18 ± 4.1 | 19.6 ± 3.5 | 18.4 ± 4.2 | 19.7 ± 4.9 | 19.5 ± 4.6 | 19.0 ± 4.2 | 20.9 ± 4.8 | 19.3 ± 3.8 |
| Total carbohydrate (g/d) | 295 ± 19 | 304 ± 23 | 285 ± 20 | 289 ± 25 | 248 ± 18 | 250 ± 24 | 252 ± 17 | 245 ± 22 |
| Total carbohydrate (% energy) | 48.5 ± 6.9 | 50.8 ± 7.1 | 47.4 ± 7.2 | 50.5 ± 7.0 | 45.5 ± 9.8 | 49.0 ± 7.2 | 45.7 ± 10.1 | 49.3 ± 7.3 |
| Total fat (g/d) | 81.5 ± 21 | 77.3 ± 27 | 83.5 ± 23 | 78.0 ± 26 | 77.0 ± 22 | 71.0 ± 25 | 70.0 ± 17 | 66.0 ± 19 |
| Total fat (% energy) | 31.9 ± 7.6 | 28.8 ± 6.9 | 32.5 ± 6.9 | 29.0 ± 6.4 | 32.6 ± 7.0 | 31.4 ± 6.5 | 31.8 ± 9.2 | 30.1 ± 6.3 |
3.1. Irisin measurement
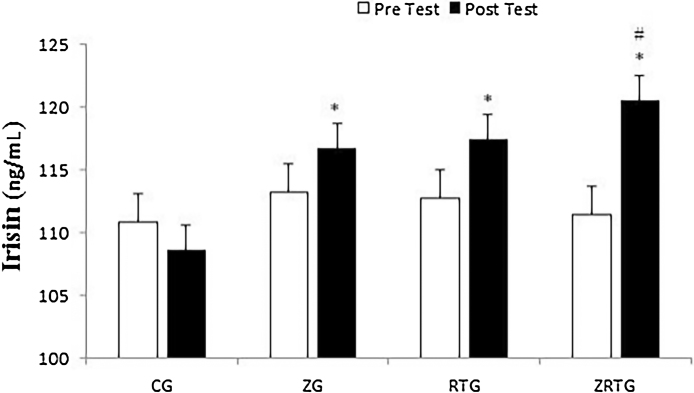
No significant differences in serum irisin levels were noted between the groups at baseline (p = 0.12). There was a significant difference in the response to the intervention between the groups (p < 0.05), with a significant increase in ZRTG when compared to the CG (p < 0.05). However, no significant differences were measured in the change between the other groups (ZG and RTG) (p > 0.05). In addition, a significant increase was noted from pre- to post-supplementation for ZG (p < 0.01, 3.09%), RTG (p < 0.001, 4.14%), and ZRTG (p < 0.001, 8.07%) (Fig. 2).
Fig. 2.
Serum irisin in pre- and post-test. Data are expressed as means ± SD. *p < 0.05 versus pretest in same group. #p < 0.05 versus CG.
3.2. Antioxidant status
No significant differences in MDA, GSH or TAC were measured at baseline between the CG, ZG, RTG or ZRTG (Table 4). However, a significant decrease in MDA levels were observed in the RTG and ZRTG groups (p < 0.05 and p < 0.001, respectively). Similarly, a significant increase in GSH was measured for the ZG (p = 0.001), RTG (p < 0.01) and ZRTG (p < 0.001). There was no significant difference in TAC between groups (Table 4).
Table 4.
Antioxidant Results (Mean ± SD) of Study Groups at Baseline and Endpoint
| Variable | Group | Baseline | Endpoint | MD | 95%CI | Sig (p) | MD | 95%CI | Sig (p) | MD | 95%CI | Sig (p) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA (nmol/mL) | CG | 1.62 ± 0.28 | 1.54 ± 0.17 | |||||||||
| ZG | 1.65 ± 0.45 | 1.16 ± 0.21 | −0.18 | −0.40 to 0.05 | 0.203 | |||||||
| RTG | 1.56 ± 0.44 | 1.08 ± 0.19 | −0.26 | −0.49 to −0.04 | 0.013 | −0.08 | −0.31 to 0.14 | 1 | ||||
| ZRTG | 1.54 ± 0.22 | 0.87 ± 0.14 | −0.38 | −0.60 to −0.15 | 0.000 | −0.20 | −0.42 to 0.02 | 0.110 | −0.12 | −0.34 to 0.11 | 1 | |
| GSH (mg/mL) | CG | 237 ± 23 | 230 ± 24 | |||||||||
| ZG | 246 ± 12 | 267 ± 19 | 23.06 | 7.32 to 38.79 | 0.001 | |||||||
| RTG | 231 ± 22 | 279 ± 19 | 21.85 | 6.11 to 37.59 | 0.002 | −1.21 | −16.9 to 14.5 | 1 | ||||
| ZRTG | 241 ± 23 | 282 ± 17 | 28.49 | 12.75 to 44.22 | 0.000 | 5.43 | −10.3 to 21.2 | 1 | 6.64 | −9.10 to 22.37 | 1 | |
| TAC (mmol/L) | CG | 520 ± 101 | 493 ± 77 | |||||||||
| ZG | 510 ± 79 | 562 ± 84 | 29.9 | −36.2 to 95.9 | 1 | |||||||
| RTG | 506 ± 110 | 566 ± 90 | 30.1 | −36.0 to 96.1 | 1 | 0.2 | −65.8 to 66.2 | 1 | ||||
| ZRTG | 500 ± 71 | 627 ± 52 | 57.0 | −9.0 to 123.0 | 0.133 | 27.1 | −38.9 to 93.2 | 1 | 26.9 | −39.1 to 93.0 | 1 |
Data presented as mean ± SD. MDA denotes malondialdehyde, GSH denotes glutathione, TAC denotes total antioxidant capacity.
4. Discussion
Our major findings were that irisin levels significantly increased following eight weeks of combined circuit resistance training and supplementation with Z. multiflora compared to the CG in apparently healthy post-menopausal women. Further findings were that resistance training, supplementation with Z. multiflora and the combination of resistance training and supplementation resulted in significant increases in irisin levels within the groups. MDA levels were significantly reduced in the RTG and ZRTG when compared to the CG; however MDA was not affected by Z. multiflora alone. GSH levels were significantly increased in the ZG, RTG, and ZRTG when compared to the CG. TAC did not change during baseline or the intervention.
It has been reported that an acute bout of exercise increases oxidants and decreases antioxidants pushing the balance toward oxidative stress.27 In contrast chronic resistance exercise training has been reported to provide protective effects against oxidative stress28 while eight weeks of combined endurance and resistance training decreased oxidative stress and increased enzymatic and non-enzymatic antioxidant capacity in untrained males.29 In this context, we measured significant changes in the antioxidants GSH and MDA (but not TAC) in response to exercise and Z. multiflora supplementation. GSH levels increased in the ZG, RTG and ZRTG when compared to the CG, while MDA levels decreased in the RTG and ZRTG, but not in the ZG alone.
GSH plays an important role in countering the oxidative-stress induced lipid peroxidation, which occurs during intense exercise.30 The increase in GSH in the RTG suggests an adaptation of the tissue-antioxidant defense system in response to exercise in these participants.31 An increase in GSH by Z. multiflora in the ZG alone suggests that this plant potentially has a protective effect against oxidative stress. This study corroborates with similar research where Z. multiflora showed strong biological antioxidant activity, and nitric oxide and malondialdehyde scavenging properties in chemical analysis.32 Moreover, recent studies in male rats evaluated the protective effects of Z. multiflora on cisplatin-induced hepatotoxicity and hepatic oxidative damage 33. Rats given a methanolic extract from Z. multiflora orally for seven days had increased levels of GSH that reduced cisplatin-induced oxidative stress. Similarly, studies in adult mice assessing the effect of Z. multiflora on hyperglycemia and blood glucose levels, lipid profile, and oxidative stress status reported significant decreases in glucose, total cholesterol, and MDA that were concomitant with an increase in GSH levels.34
We observed a significant reduction in the levels of MDA in the RTG and ZRTG, but not in the ZG alone. MDA is a common marker of lipid peroxidation and oxidative stress and levels of MDA are reported to increase following acute intense exercise.35 These data suggest that chronic exercise may have a greater advantage in protection against the effects of oxidative stress. These findings corroborate with Çakir-Atabek et al. (2010) where chronic resistance exercise training in men resulted in significantly reduced MDA levels, increased GSH levels, and therefore suggests a protective mechanism against oxidative stress.28 It is unclear why ZG alone did not reduce the levels of MDA, but it is interesting that the mean decrease in MDA in ZG was approximately 75% of that observed in the RTG group, and that MDA in the ZRTG group decreased by 25% more than RTG alone. It is possible therefore, that the lack of significance in the ZG group and in ZRTG compared to RTG was due to a smaller relative effect of Z. multiflora on antioxidant status than exercise, and that these differences would have become statistically significant with a larger sample population. This needs further investigation in future studies.
Our analysis of TAC found no significant changes between any of the groups; however, a trend was observed where TAC increased in the ZG, RTG and ZRTG, which may reflect enhanced antioxidant defenses in response to exercise-induced oxidative stress. This finding is in accordance with previous studies that showed increased plasma TAC levels across a range of exercise modalities.36, 37, 38
A potential mechanism by which these protective benefits may occur are through increased production and release of muscle-derived secretory factors or myokines. Myokines facilitate tissue-specific crosstalk between skeletal muscle and other tissues such as liver and adipose tissue.39 Myokines possess important anti-inflammatory and metabolic properties and play a crucial role in muscle adaptation to exercise.40 Previously irisin was defined as a myokine that is responsive to exercise,41, 42 negatively correlated with malondialdehyde,13 and improves endothelial dysfunction via decreasing oxidative stress in type 2 diabetes.43
Nevertheless, several studies have failed to show increases in irisin levels in plasma after chronic training,44, 45 and reduced plasma levels of irisin after intermittent sprint running has also been demonstrated.44 Norheim et al. (2014) reported that circulating irisin was reduced in response to 12 weeks of training, but was increased after acute exercise in men.41 Conversely, others reported that acute strength training for up to 30 minutes did not increase serum levels of irisin.45 Interestingly, the only study to our knowledge to previously examine the effect of exercise on irisin levels in post-menopausal women found similar results to the current study following an eight-week aerobic exercise intervention.15 The reasons for these contradictory results are not known but may relate to differences in the cohort studied.
Irisin levels significantly increased following Z. multiflora supplementation. Previously Zataria has been shown to exhibit radical-scavenging activity and protection of human lymphocytes from the genetic damage and side-effects induced by exposure to radiation.8 Therefore, we believe the increases in irisin levels following eight weeks of supplementation with Z. multiflora are due in part to positive effects of Z. multiflora on antioxidative enzyme activities, resulting in reduced oxidative stress, mainly through promoting enzymatic and non-enzymatic antioxidant capacity. These data support previous work indicating a relationship between irisin and antioxidant capacity.11, 13 Whether irisin is a vital molecule involved in up-regulation of antioxidant capacity or the increase is secondary to improved antioxidant capacity, is an important issue, which should be considered in future research. Similarly, it is not clear how Z. multiflora increased irisin levels, and no reports to our knowledge exist on the mechanism(s) by which this might occur. However, as previously noted, irisin can be activated by PGC-1α10, and this transcriptional coactivator can activate peroxisome proliferating activator receptor gamma (PPARγ).46 Previously, research demonstrated that Z. multiflora had a direct insulin-like effect, increased adiponectin, and activated PPARγ protein expression which was concomitant with improved antihyperglycemic effects and improved insulin sensitivity in insulin-resistant rats.47 Accordingly, it is an intriguing notion that perhaps Z. multiflora could act through the PGC-1α/PPARγ transcriptional network to increase irisin levels. However, this is yet to be tested.
Another novel finding of the current study was that serum levels of irisin significantly increased after eight weeks of combined circuit resistance training and supplementation with Z. multiflora. However, increases in irisin levels in the combined treatment were not significantly greater than either the exercise or the herbal treatment alone, indicating no additive benefits in post-menopausal women. Our findings are supported by those of a previous study48 who failed to find any positive additive effect of combined antioxidant supplementation and exercise training on cognitive function.48 There are, however, some contradictory findings regarding positive additive effects of combined antioxidant supplementation and exercise training on various body organs and/or systems. In animals, an additive effect of combined antioxidant supplementation (i.e., vitamin E and Omega-3 fatty acids) and exercise training (i.e., swimming) has previously been observed to increase antioxidant activity in brain tissue49 and improve synaptic plasticity49, 50 which is inconsistent with findings of the current study. As mentioned earlier, both exercise training (chronic) and Z. multiflora have been reported to promote antioxidant capacity. Therefore, the combination of antioxidant supplementation and exercise training is expected to boost antioxidant capacity and potentially could lead to a greater improvement than either intervention separately. The lack of an additive effect on irisin levels in the current study may be due in part to reaching a maximum ceiling of antioxidant capacity during each intervention. Further studies are needed to confirm these findings.
It is important to note the limitations of the study. Although compounds from Z. multiflora have been used in several studies, we do not have the information to determine exactly which compounds (or combination thereof) are causing the observed effects. Similarly, while we measured irisin in serum, it is not clear whether the irisin we measured was produced and secreted from skeletal muscle. It is tempting to speculate that the irisin measured in the current study is muscle-specific based on studies by Boström et al. (2012) that showed exercise induced the expression of PGC-1α that subsequently increased irisin expression and secretion from skeletal muscle cells.14
Another potential limitation is that related factors to myokines, such as insulin, brain-derived neurotrophic factor, and Interleukin-6 were not assessed in this study. Additionally, while ELISA is widely used to detect and quantify biological molecules, it cannot be ruled out that non-specific cross-reactivity with other proteins might occur. The specificity information provided with the ELISA kit (CUSABIO) indicated high and excellent sensitivity for detection of human irisin and has been reported previously.51, 52, 53, 54 Ideally, it would be good practice to confirm non-specific cross-reactivity in these studies by immunoblotting for the expected molecular weight of irisin and/or the use of recombinant irisin and size comparison. Unfortunately, there was insufficient sample available to perform these additional studies in this case and consequently the potential for cross-reactivity must be acknowledged as a potential confounder.
The present study provided some evidence of positive effects of circuit resistance training as well as Z. multiflora supplementation on the expression of the novel myokine, irisin, within post-menopausal women presumably through antioxidant pathways. The interaction between the expression of myokines and antioxidant pathways need to be the subject of more detailed studies.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Velders M., Diel P. How sex hormones promote skeletal muscle regeneration. Sports Med. 2013;43:1089–1100. doi: 10.1007/s40279-013-0081-6. [DOI] [PubMed] [Google Scholar]
- 2.Richardson S.J. 1. The biological basis of the menopause. Baillieres Clin Endocrinol Metab. 1993;7:1–16. doi: 10.1016/s0950-351x(05)80267-8. [DOI] [PubMed] [Google Scholar]
- 3.Bednarek-Tupikowska G., Tupikowski K., Bidzińska B., Bohdanowicz-Pawlak A., Antonowicz-Juchniewicz J., Kosowska B. Serum lipid peroxides and total antioxidant status in postmenopausal women on hormone replacement therapy. Gynecol Endocrinol. 2004;19:57–63. doi: 10.1080/09513590412331272328. [DOI] [PubMed] [Google Scholar]
- 4.Kendall K.L., Fairman C.M. Women and exercise in aging. J Sport Health Sci. 2014;3:170–178. [Google Scholar]
- 5.Ohlendieck K. Proteomic identification of biomarkers of skeletal muscle disorders. Biomark Med. 2013;7:169–186. doi: 10.2217/bmm.12.96. [DOI] [PubMed] [Google Scholar]
- 6.Tresoldi I., Foti C., Masuelli L., Frajese G.V., Rossi P., Modesti A. Effects of dragon boat training on cytokine production and oxidative stress in breast cancer patients: a pilot study. Open J Immunol. 2014:2014. [Google Scholar]
- 7.Saei-Dehkordi S.S., Tajik H., Moradi M., Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinimehr S.J., Mahmoudzadeh A., Ahmadi A., Ashrafi S.A., Shafaghati N., Hedayati N. The radioprotective effect of Zataria multiflora against genotoxicity induced by γ irradiation in human blood lymphocytes. Cancer Biother Radiopharm. 2011;26:325–329. doi: 10.1089/cbr.2010.0896. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Li R., Meng Y., Li S., Donelan W., Zhao Y. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 11.Huh J.Y., Mantzoros C.S. Irisin physiology, oxidative stress, and thyroid dysfunction: what next? Metabol-Clin Exp. 2015;64:765–767. doi: 10.1016/j.metabol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Samy D.M., Ismail C.A., Nassra R.A. Circulating irisin concentrations in rat models of thyroid dysfunction—effect of exercise. Metabolism. 2015;64:804–813. doi: 10.1016/j.metabol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Belviranli M., Okudan N., Çelik F. Association of circulating irisin with insulin resistance and oxidative stress in obese women. Horm Metabol Res. 2016;48:653–657. doi: 10.1055/s-0042-116155. [DOI] [PubMed] [Google Scholar]
- 14.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-[agr]-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto-Mikami E., Sato K., Kurihara T., Hasegawa N., Fujie S., Fujita S. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLOS ONE. 2015;10:e0120354. doi: 10.1371/journal.pone.0120354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brentano M.A., Cadore E.L., Da Silva E.M., Ambrosini A.B., Coertjens M., Petkowicz R. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Streng Condit Res. 2008;22:1816–1825. doi: 10.1519/JSC.0b013e31817ae3f1. [DOI] [PubMed] [Google Scholar]
- 17.Howe T.E., Shea B., Dawson L.J., Downie F., Murray A., Ross C. Exercise for preventing and treating osteoporosis in postmenopausal women. Coch Libr. 2011 doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Williams A.D., Ahuja K.D., Almond J.B., Robertson I.K., Ball M.J. Progressive resistance training might improve vascular function in older women but not in older men. J Sci Med Sport. 2013;16:76–81. doi: 10.1016/j.jsams.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Draper M.W., Flowers D.E., Huster W.J., Neild J.A., Harper K.D., Arnaud C. A controlled trial of raloxifene (LY139481) HCl: impact on bone turnover and serum lipid profile in healthy postmenopausal women. J Bone Miner Res. 1996;11:835–842. doi: 10.1002/jbmr.5650110615. [DOI] [PubMed] [Google Scholar]
- 20.Boss S.M., Huster W.J., Neild J.A., Glant M.D., Eisenhut C.C., Draper M.W. Effects of raloxifene hydrochloride on the endometrium of postmenopausal women. Am J Obstetr Gynecol. 1997;177:1458–1464. doi: 10.1016/s0002-9378(97)70091-7. [DOI] [PubMed] [Google Scholar]
- 21.Brzycki M. Strength testing—predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Dance. 1993;64:88–90. [Google Scholar]
- 22.Ghanbari-Niaki A., Saeidi A., Aliakbari-Beydokhti M., Ardeshiri S., Kolahdouzi S., Chaichi M.J. Effects of circuit resistance training with crocus sativus (saffron) supplementation on plasma viscosity and fibrinogen. Ann Appl Sport Sci. 2015;3:1–10. [Google Scholar]
- 23.Sheikholeslami V.D., Ahmadi K.G.F. Changes in antioxidant status and cardiovascular risk factors of overweight young men after six weeks supplementation of whey protein isolate and resistance training. Appetite. 2012;59:673–678. doi: 10.1016/j.appet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24.McCance R. Royal Society of Chemistry; Cambridge: 2002. Food Standards Agency; AFRC Institute of Food Research McCance and Widdowson's the composition of foods. [Google Scholar]
- 25.Wasowicz W., Neve J., Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–2526. [PubMed] [Google Scholar]
- 26.Benzie I.F., Szeto Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem. 1999;47:633–636. doi: 10.1021/jf9807768. [DOI] [PubMed] [Google Scholar]
- 27.Kurkcu R. The effects of short-term exercise on the parameters of oxidant and antioxidant system in handball players. Afr J Pharm Pharmacol. 2010;4:448–452. [Google Scholar]
- 28.Çakir-Atabek H., Demir S., PinarbaSili R.D., Gündüz N. Effects of different resistance training intensity on indices of oxidative stress. J Streng Cond Res. 2010;24:2491–2497. doi: 10.1519/JSC.0b013e3181ddb111. [DOI] [PubMed] [Google Scholar]
- 29.Azizbeigi K., Azarbayjani M.A., Atashak S., Stannard S.R. Effect of moderate and high resistance training intensity on indices of inflammatory and oxidative stress. Res Sports Med. 2015;23:73–87. doi: 10.1080/15438627.2014.975807. [DOI] [PubMed] [Google Scholar]
- 30.Kerksick C., Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol Cell Biochem. 1999;196:31–42. [PubMed] [Google Scholar]
- 32.Karimian P., Kavoosi G., Amieghofran Z., Kalantar F. Reduction of NADH oxidase, NO synthase, TNFα, and IL-1β mRNA expression levels on lipopolysacharide-stimulated murine macrophages by Zataria multiflora. Mol Biol Res Commun. 2013;2:89–100. [Google Scholar]
- 33.Ahmadipour A., Sharififar F., Nakhaipour F., Samanian M., Karami-Mohajeri S. Hepatoprotective effect of Zataria multiflora Boisson cisplatin-induced oxidative stress in male rat. J Med Life. 2015;8:275. [PMC free article] [PubMed] [Google Scholar]
- 34.Samarghandian S., Azimini-Nezhad M., Farkhondeh T. The effects of Zataria multiflora on blood glucose, lipid profile and oxidative stress parameters in adult mice during exposure to bisphenol A. Cardiovasc Haematol Disord Drug Targets. 2016;16:41–46. doi: 10.2174/1871529x16666160531111106. [DOI] [PubMed] [Google Scholar]
- 35.Kiyici F., Kishali N. Acute effect of intense exercises on serum superoxide dismutase, catalase and malondialdehyde levels in soccer players. J Sports Med Phys Fitness. 2012;52:107–111. [PubMed] [Google Scholar]
- 36.Cipryan L. IL-6, antioxidant capacity and muscle damage markers following high-intensity interval training protocols. J Hum Kinet. 2017;56:139–148. doi: 10.1515/hukin-2017-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norouziyan S., Shemshaki A., Hanachi P. The effect of eccentric exercise on total anti-oxidant capacity, reduced glutathione and malondialdehyde levels in active women. Zahedan J Res Med Sci. 2014;16:47–52. [Google Scholar]
- 38.Gökhan İ., Aktaş Y., Arikan G. 2014. A comparison of antioxidant capacity and some lipoprotein values in swimmers and sedentary subjects. [Google Scholar]
- 39.Hartwig S., Raschke S., Knebel B., Scheler M., Irmler M., Passlack W. Secretome profiling of primary human skeletal muscle cells. Biochim Biophys Acta (BBA)-Prot Proteom. 2014;1844:1011–1017. doi: 10.1016/j.bbapap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Scheele C., Nielsen S., Pedersen B.K. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 42.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu D., Wang H., Zhang J., Zhang X., Xin C., Zhang F. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–147. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pekkala S., Wiklund P.K., Hulmi J.J., Ahtiainen J.P., Horttanainen M., Pöllänen E. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang H., Ward W.F. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 47.Mohammadi A., Gholamhoseinian A., Fallah H. Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17:263. [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhari K., Wong J.M., Vann P.H., Sumien N. Exercise training and antioxidant supplementation independently improve cognitive function in adult male and female GFAP-APOE mice. J Sport Health Sci. 2014;3:196–205. [Google Scholar]
- 49.Devi S.A., Kiran T.R. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 50.Wu A., Ying Z., Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M., Yang M., Zhou X., Fang X., Hu W., Zhu W. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:1485–1493. doi: 10.1210/jc.2014-2544. [DOI] [PubMed] [Google Scholar]
- 52.Moienneia N., Hosseini S.R.A. Acute and chronic responses of metabolic myokine to different intensities of exercise in sedentary young women. Obes Med. 2016;1:15–20. [Google Scholar]
- 53.Li H., Xu X., Wang X., Liao X., Li L., Yang G. Free androgen index and Irisin in polycystic ovary syndrome. J Endocrinol Investig. 2016;39:549–556. doi: 10.1007/s40618-015-0403-7. [DOI] [PubMed] [Google Scholar]
- 54.Aydin S., Aydin S., Kobat M.A., Kalayci M., Eren M.N., Yilmaz M. Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides. 2014;56:141–145. doi: 10.1016/j.peptides.2014.04.002. [DOI] [PubMed] [Google Scholar]