Figure 3.
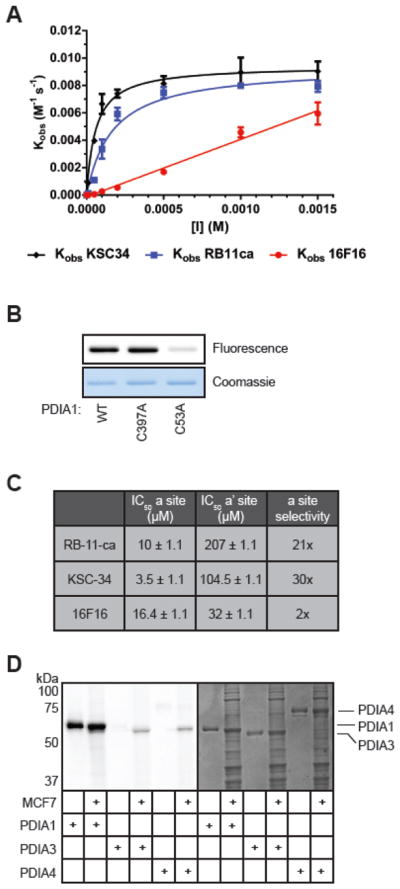
Evaluation of potency and selectivity of KSC-34. (A) Concentration and time-dependent inhibition of PDIA1 by KSC-34, RB-11-ca, and 16F16. PDIA1 reductase activity was measured using an insulin reduction assay in a 100 μL reaction volume containing 0.5 μM PDIA1, 0.16 mM insulin, and 1 mM DTT in assay buffer. Turbidity of the insulin solution was measured over time at various concentrations and pre-incubation times with KSC-34. Error bars represent SD from n=2 experiments. (B) Labeling of WT, C397A and C53A by KSC-34. Each recombinant PDIA1 protein (50 μg/ml) was incubated with KSC-34 (5 μM) for 1 hour, and subjected to CuAAC with TAMRA-azide prior to in-gel fluorescence. (C) Active-site selectivity for KSC-34, RB-11-ca, and 16F16. KSC-34 was found to have a 30-fold selectivity for the a domain over the a′ domain, compared to RB-11-ca which exhibited a 21-fold selectivity and 16F16 which exhibited 2-fold selectivity. Error is calculated by PRISM as +/− SEM from n=3 experiments. (D) PDI isoform selectivity. KSC-34 was found to only covalently modify PDIA1, over PDIA3 and PDIA4, two closely related family members. Fluorescent bands in cell lysate lanes for PDIA3 and PDIA4 samples represent endogenous PDIA1 protein.
