Abstract
OBJECTIVE: A previous phase-2 trial to assess the addition of Endostar to gemcitabine and cisplatin (GC) chemotherapy showed that it improves prognosis in metastatic nasopharyngeal carcinoma (M-NPC) but the study cohort was small. We wished to update that phase-2 trial by enrolling an additional 44 patients and to assess the benefit of Endostar+GC chemotherapy. METHODS: An analysis of 72 M-NPC patients treated between July 2010 and November 2016 was done. The treatment regimen was a combination of gemcitabine (1,000 mg/m2) on days 1 and 8, cisplatin (80 mg/m2) on day 1, and Endostar (15 mg/day) from day 1 to day 14 of a 21-day cycle for ≥2 cycles. The acute toxic effects and therapeutic efficacy were analyzed. RESULTS: The response rate was 77.8%. The median progression-free and overall survivals were 12 and 19.5 months, respectively. A total of 329 cycles of GC and 288 cycles of Endostar were delivered to 72 patients, with the median number of four (range, 2–10) cycles administered per patient. The main grade-3/4 hematologic toxicities were leukopenia (54.1%) and neutropenia (59.8%). The number of non-hematologic adverse events was minimal. The regimen was well-tolerated. CONCLUSIONS: Endostar+GC chemotherapy is an effective, well-tolerated regimen for M-NPC.
Introduction
Nasopharyngeal carcinoma (NPC) has an extremely unbalanced distribution in China: the highest incidence is in southern China, particularly in Guangdong Province [1]. NPC is highly sensitive to chemotherapy and radiotherapy. Due to advances in ionizing radiation such as intensity-modulated radiotherapy, imaging such as magnetic resonance imaging (MRI), and chemoradiotherapy, the local and regional control rates reached ≈90% at 3 years after treatment for stage III–IVB NPC [2], [3], [4]. However, ≈20% of patients with stage-III–IVB NPC fail optimal treatment due to metastasis [2], [5].
The standard treatment for patients with metastatic nasopharyngeal carcinoma (M-NPC) is platinum-based doublet chemotherapy [6], [7]. In 2016, Zhang et al. [8] presented the results of the first randomized, multicenter, open-label, phase-3 trial comparing the efficacy and safety of gemcitabine plus cisplatin (GC) versus fluorouracil plus cisplatin in patients with recurrent or M-NPC. Their results suggested that GC is more effective than fluorouracil plus cisplatin in the treatment of recurrent NPC or M-NPC. Those results established GC as standard first-line treatment for this population. However, the median time to progression remained relatively constant at 7 months in the GC group, which was similar to that for historical controls of two-drug regimens [9], [10].
One strategy to improve overall survival (OS) in patients with M-NPC is to combine molecular-targeted agents and common cytotoxic agents. In our previous phase-2 trial, we found that the median PFS was 19.4 (95% confidence interval, 13.6–25.1) months and 1-year PFS was 69.8% for 28 NPC patients with metachronous metastasis who received recombinant human endostatin (Endostar; a recombinant endostatin with an additional nine amino-acid sequence at the N terminal of the protein and a six-histidine tag) in combination with a standard GC regimen [11]. Whether this survival benefit is durable for a larger population is not known. We used the same regimen for treating an additional 44 patients. Here, we report the results for the 72 (i.e., 28 + 44) patients with M-NPC who received Endostar in combination with a standard GC regimen.
Results
Patient Characteristics
Between July 2010 and November 2016, 72 patients with M-NPC received GC+Endostar chemotherapy at Zhejiang Cancer Hospital. The characteristics of the patients are summarized in Table 1. The patients were predominantly male (76.4%) and the median age of all patients was 47 (range, 18–64) years. 29.2% patients had distant metastasis at onset. The sites of metastasis were bone, liver, lungs, and distant lymph nodes; among them, the lungs were the most common site (44.4%). Also, 55.6% of patients had one metastatic organ and 20.8% of patients had one metastatic site. The date of last follow-up was March 1, 2017.
Table 1.
Clinical Characteristics of Study Participants
| Characteristic | No. Patients | Percentage |
|---|---|---|
| Total | 72 | 100 |
| Sex | ||
| Male | 55 | 76.4 |
| Female | 17 | 23.6 |
| Median age (range); years | 47 (18–64) | |
| Median Karnofsky performance status (range); % | 80.0 (70–100) | |
| Synchronous metastasis | ||
| Yes | 21 | 29.2 |
| No | 51 | 70.8 |
| Metastatic sites | ||
| Lung | 32 | 44.4 |
| Liver | 29 | 40.3 |
| Bone | 31 | 43.1 |
| Lymph nodes (extra-regional) | 17 | 23.6 |
| Number of metastatic organs | ||
| 1 | 40 | 55.6 |
| ≥2 | 32 | 44.4 |
| Number of metastatic sites | ||
| 1 | 15 | 20.8 |
| ≥2 | 57 | 79.2 |
Treatment Efficacy
A total of 329 cycles of GC and 288 cycles of Endostar were delivered to 72 patients. The treatment cycles were as follows: 2, 45, 21 and 4 patients received 2, 3-4, 5-6 and >6 cycles of GC, respectively; 10, 48, 11 and 3 patients received 2, 3-4, 5-6 and >6 cycles of Endostar, respectively. The median number of cycles administered per patient was four (range, 2–10). A total of 16 patients (22.2%) had a CR, 40 patients (55.6%) had a PR, 6 patients (8.3%) had SD, and 10 patients (13.9%) had PD. The overall response rate (ORR) was 77.8%, and the disease control rate was 86.1%. With a median follow-up of 19.5 (range, 8–84) months, the median PFS was 12 months and the median OS for all patients was 19.5 months. For the entire group, the 1-, 2-, and 3-year PFS was 45.4%, 26.7%, and 23.3% (Figure 1) and the 1-, 2-, and 3-year OS was 87.4%, 54.2%, and 31.9%, respectively (Figure 2). PFS benefit was observed for the previously included 28 patients compared to the recently included 44 patients (1-year 57.1% vs. 37.9%, 2-year 39.3% vs. 18.6%, 3-year 35.7% vs. 14.0%, P = .040). OS benefit was not observed for the previously included 28 patients compared to the recently included 44 patients (1-year 85.5% vs. 88.4%, 2-year 64.3% vs. 45.3%, 3-year 42.9% vs. 21.2%, P = .139).
Figure 1.
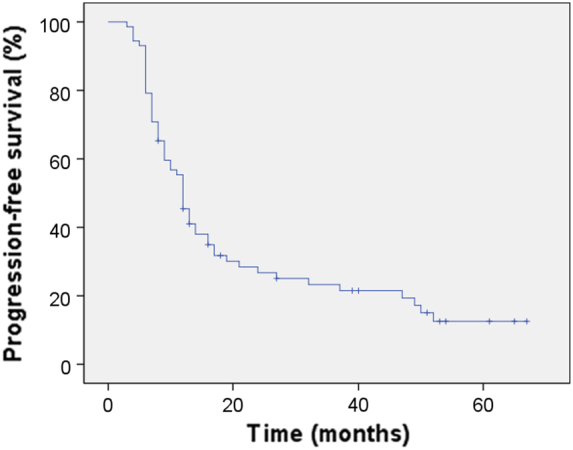
Kaplan–Meier Curve of progression-free survival.
Figure 2.
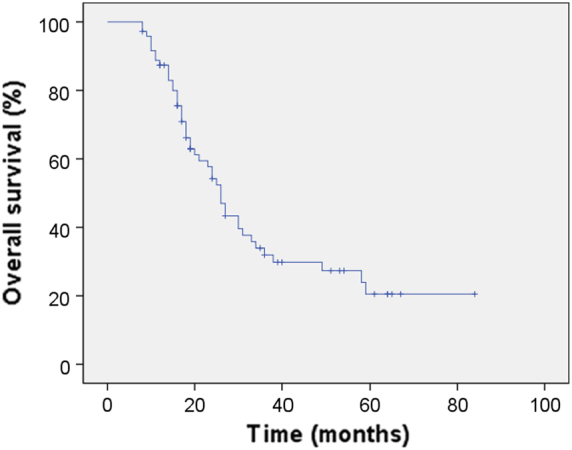
Kaplan–Meier Curve of overall survival.
Toxicity of Treatment
Table 2 summarizes the adverse events most associated with the study drugs. The most common hematologic toxicities were leukopenia (97.2%), neutropenia (95.8%), anemia (84.7%) and thrombocytopenia (80.6%). The main grade-3/4 hematologic toxicities were leukopenia (54.1%) and neutropenia (59.8%). All patients received treatment with granulocyte colony-stimulating factor (G-CSF) or interleukin-11/recombinant human thrombopoietin interleukin (IL-11/rhTPO) in response to grade-3 or -4 hematologic adverse events. No patient developed grade-3/4 kidney dysfunction but four (5.6%) patients developed grade-1/2 kidney dysfunction. Eight (11.1%) patients developed grade-3/4 liver dysfunction and 40 (55.6%) patients developed grade-1/2 liver dysfunction. The most common non-hematologic events were nausea (25%), vomiting (20.8%), anorexia (20.8%) and fatigue (9.7%). The main grade-3/4 non-hematologic toxicities were nausea (4.2%), vomiting (4.2%) and anorexia (2.8%). One patient had grade-2 left ventricular diastolic dysfunction. No treatment-related death occurred during our study.
Table 2.
Adverse Events Related to the Gemcitabine-Cisplatin + Endostar Regimen
| Toxicity |
Any Grade |
Grade 3 |
Grade 4 |
|---|---|---|---|
| No. Patients (%) | No. Patients (%) | No. Patients (%) | |
| Biologic adverse events | |||
| Leukopenia | 70 (97.2) | 34 (47.2) | 5 (6.9) |
| Neutropenia | 69 (95.8) | 31 (43.1) | 12 (16.7) |
| Anemia | 61 (84.7) | 11 (15.3) | 6 (8.3) |
| Thrombocytopenia | 58 (80.6) | 13 (18.1) | 10 (13.9) |
| Liver dysfunction | 48 (66.7) | 7 (9.7) | 1 (1.4) |
| Kidney dysfunction | 4 (5.6) | 0 | 0 |
| Clinical adverse events | |||
| Fatigue | 7 (9.7) | - | - |
| Nausea | 22 (25) | 3 (4.2) | - |
| Vomiting | 15 (20.8) | 3 (4.2) | - |
| Stomatitis (mucositis) | 2 (2.8) | - | - |
| Diarrhea | 2 (2.8) | - | - |
| Anorexia | 15 (20.8) | 2 (2.8) | - |
Prognostic Factors for PFS
Factors that were considered for analyses were related to patients (age, sex, smoking, alcohol consumption), disease (synchronous metastasis, number of metastatic organs, number of metastatic sites, specific metastatic sites), and treatment (number of cycles of GC chemotherapy, number of cycles of Endostar therapy). P < .1 was used as the cutoff value of significance for variable selection in univariable modeling to avoid missing potentially important prognostic factors.
Significant alcohol consumption (P = .069), liver metastases (P < .001), Endostar cycles >4 (P = .034), metastatic sites >1 (P = .001) and metastatic organs >1 (P = .001) were negative prognostic factors (Table 3). Cox multivariate analyses indentified the number of metastatic sites (P = .001) to be an independent positive prognostic factor for PFS (Table 4). A PFS benefit was observed for patients without liver metastases compared with patients with liver metastases in multivariate analyses, though this difference was not significant (P = .053) (Table 4).
Table 3.
The Relationship Between Clinicopathological Variables With Progression-Free Survival (PFS) in Patients With Metastatic Nasopharyngeal Carcinoma
| Feature | No. of Patients | PFS (%) |
χ2 | Pa | ||
|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | ||||
| Sex | 0.053 | 0.817 | ||||
| Female | 17 (23.6%) | 52.9 | 33.6 | 33.6 | ||
| Male | 55 (76.4%) | 43.0 | 24.3 | 19.7 | ||
| Age (years)b | 0.125 | 0.724 | ||||
| <48 | 37 (51.4%) | 48.6 | 26.3 | 18.8 | ||
| ≥48 | 35 (48.6%) | 41.9 | 26.9 | 26.9 | ||
| Synchronous metastasis | 1.491 | 0.222 | ||||
| No | 51 (70.8%) | 46.4 | 32.2 | 27.3 | ||
| Yes | 21 (29.2%) | 42.9 | 14.3 | 14.3 | ||
| Smoking indexc | 0.768 | 0.381 | ||||
| ≤100 | 37 (51.4%) | 54.1 | 28.8 | 25.2 | ||
| >100 | 35 (48.6%) | 36.1 | 24.1 | 21.1 | ||
| Alcohol consumption | 3.317 | 0.069⁎ | ||||
| No | 51 (70.8%) | 52.9 | 31.0 | 26.2 | ||
| Yes | 21 (29.2%) | 26.2 | 15.7 | 15.7 | ||
| Number of metastatic organs | 11.262 | 0.001** | ||||
| Oligo | 40 (55.6%) | 65.0 | 43.1 | 36.7 | ||
| Multiple | 32 (44.4%) | 19.9 | 6.6 | 6.6 | ||
| Number of metastatic sites | 11.267 | 0.001** | ||||
| Oligo | 15 (20.8%) | 80.0 | 66.0 | 58.7 | ||
| Multiple | 57 (79.2%) | 36.2 | 15.8 | 13.5 | ||
| Chemotherapy cycles | 0.770 | 0.380 | ||||
| ≤4 | 47 (65.3%) | 50.4 | 28.6 | 26.0 | ||
| >4 | 25 (34.7%) | 36.0 | 23.3 | 17.5 | ||
| Endostar cycles | 4.472 | 0.034* | ||||
| ≤4 | 58 (80.6%) | 47.7 | 30.5 | 28.4 | ||
| >4 | 14 (19.4%) | 35.7 | 10.7 | 0 | ||
| Lung metastasis | 0.710 | 0.440 | ||||
| Absent | 40 (55.6%) | 44.2 | 23.8 | 23.8 | ||
| Present | 32 (44.4%) | 46.9 | 30.0 | 22.5 | ||
| Liver metastasis | 13.078 | <0.001** | ||||
| Absent | 43 (59.7%) | 60.5 | 42.5 | 36.6 | ||
| Present | 29 (40.3%) | 22.1 | 3.7 | 3.7 | ||
| Bone metastasis | 0.437 | 0.509 | ||||
| Absent | 41 (56.9%) | 43.0 | 26.9 | 20.9 | ||
| Present | 31 (43.1%) | 48.4 | 26.2 | 26.2 | ||
| Nodal metastasis | 1.604 | 0.205 | ||||
| Absent | 55 (76.4%) | 52.7 | 30.7 | 26.1 | ||
| Present | 17 (23.6%) | 19.9 | 13.2 | 13.2 | ||
Log-rank test.
Patients were divided into two groups according to the median age.
Smoking Index is defined as the number of cigarettes used per day × the total smoking time (years).
P < .1.
P < .01.
Table 4.
Multivariate Analyses of Prognostic Factors for Progress-Free Survival of 72 Patients With Metastatic NPC
| Variable | PFS |
||
|---|---|---|---|
| Odds Ratio | 95% CI | P | |
| Alcohol consumption | 1.365 | 0.759-2.455 | 0.299 |
| Liver metastases | 2.080 | 0.991-4.365 | 0.053 |
| Endostar cycles | 1.106 | 0.921-1.329 | 0.279 |
| Metastatic sites | 2.483 | 1.092-5.690 | 0.030 |
| Metastatic organs | 1.055 | 0.495-2.251 | 0.889 |
HR, hazard ratio; CI, confidence interval.
Discussion
Palliative chemotherapy is the main treatment for patients with M-NPC. The latter is highly sensitive to chemotherapy, but the OS is poor and only few patients may have long-period disease-free survival irrespective of whether a platinum-based two or triplet drug regimen is chosen. The median time to disease progression remains relatively static at 7 to 8 months [9], [10], [12]. Development of a new palliative regimen to increase disease control and prolong survival in such patients is needed.
In our previous phase-2 trial, we found that a GC+Endostar regimen elicited excellent results (median PFS, 19.4 months; 1-year PFS, 69.8%) for treating NPC with metachronous metastasis, but the study cohort was small [11]. Whether the results could be sustained for a longer duration for a larger population needed to be determined.
Here, we enrolled 72 patients and the primary endpoint was reached. The ORR was 77.8%, the median PFS was 12 months, and the 1-, 2-, and 3-year PFS was 45.4%, 26.7%, and 23.3%, respectively, for the entire group. The toxicity profiles of the GC+Endostar regimen were acceptable. Also, the toxicities were mainly hematologic and could be overcome by using G-CSF or IL-11/rhTPO. Hence, this regimen was well-tolerated and effective in patients with M-NPC.
Between 2002 and 2015, to confirm the synergistic effect of gemcitabine and platinum in vitro, four small, phase-2 trials reported the results of a GC regimen or gemcitabine plus oxaliplatin regimen in patients with recurrent NPC or M-NPC [13], [14], [15], [16]. In 2002, Ngan et al. [13] reported a response rate of 73% and a median PFS of 10.6 (range, 8.5-12.6) months for 44 patients who received salvage chemotherapy with a GC regimen. In 2008, Jialei Wang and colleagues [14] reported a response rate of 42.7% and a median PFS of 5.6 months for 75 patients who received salvage chemotherapy with a GC regimen. In 2009, Ma et al. [15] reported a response rate of 56.1% and a median PFS of 9 (range, 7.3-10) months for 42 patients who received salvage chemotherapy with a gemcitabine plus oxaliplatin regimen. In 2015, Hsieh and colleagues [16] reported a response rate of 51.9% and a median PFS of 9.8 (range, 6.5-13.0) months for 52 patients who received salvage chemotherapy with a GC regimen. In 2016, Zhang and colleagues [8] presented the results of the first randomized, multicenter, open-label, phase-3 trial comparing the efficacy and safety of GC versus fluorouracil plus cisplatin in patients with recurrent NPC or M-NPC. They reported a response rate of 64% and a median PFS of 7 (range, 4.4-10.9) months for 156 patients who received salvage chemotherapy with a GC regimen.
Hence, for patients with recurrent NPC or M-NPC who receive gemcitabine and platinum chemotherapy, response rates of ≤73% and a median PFS of ≤10.6 months can be achieved. Endostatin is an anti-vascular endothelial growth factor antibody found in cancerous and normal tissues. Walia and colleagues reported that more than one-fifth of NPC patients had increased serum levels of endostatin [17]. Endostar is a new recombinant human endostatin that can inhibit tumor growth primarily through direct inhibition of the proliferation of vascular endothelial cells and vascular normalization [18].
In the present study enrolling 72 patients with M-NPC, the GC+Endostar regimen elicited an ORR of 77.8%, a median time to progression of 12 months and 1-, 2-, and 3-year PFS of 45.4%, 26.7%, and 23.3%, respectively. Our data are exceptionally high compared with the results of the studies mentioned above using gemcitabine and platinum chemotherapy. When it comes to OS, the median OS for our entire group is lower than those reported by Li Zhang et al. [8] (19.5 vs. 29.1 months). The possible reason was that the Li Zhang’ group had high numbers of patients received second-line or third-line chemotherapy after documented progression compared to our entire group (41% vs. 30.5%). Endostar can also be used for the treatment of locally recurrent NPC. Guan and colleagues [19] conducted a retrospective study to evaluate the short-term efficacy and safety of Endostar combined with chemoradiotherapy for the treatment of advanced, locally recurrent NPC. They reported a CR of 90% and prevalence for nasopharyngeal mucosal necrosis of ≤31.8%.
Ngan et al. [13] used a combination of gemcitabine (1000 mg/m2) on days 1, 8 and 15, and cisplatin (50 mg/m2) on days 1 and 8 of a 28-day cycle. With a mean of 4.8 cycles, the grade-3/4 hematologic toxicities included neutropenia (37%), anemia (11%) and thrombocytopenia (16%). Wang and colleagues [14] used a combination of gemcitabine (1000 mg/m2) on days 1 and 8, and cisplatin (25 mg/m2) on days 1, 2 and 3 of a 21-day cycle. With a mean of 3.6 cycles, the grade-3/4 hematologic toxicities included neutropenia (8%), anemia (4%) and thrombocytopenia (4%). Here, we used a combination of gemcitabine (1000 mg/m2) on days 1 and 8, and cisplatin (80 mg/m2) on day 1 of a 21-day cycle. With a mean of 4.6 cycles, the grade-3/4 hematologic toxicities included neutropenia (59.8%), anemia (23.6%) and thrombocytopenia (32%). Hsieh and colleagues [16] used a combination of gemcitabine (1250 mg/m2) on days 1 and 8, and cisplatin (75 mg/m2) on day 1 of a 21-day cycle. With a mean of 6.6 cycles, the grade-3/4 hematologic toxicities included leukopenia (61.6%), anemia (44.2%) and thrombocytopenia (21.1%). Besides, one treatment-related death occurred during the research because of leucopenia [16]. The grade-3/4 hematologic toxicities observed in our study were similar to those documented by Hsieh and colleagues, but much higher than those of Ngan and coworkers and Wang and colleagues.
There are three explanations for the differences mentioned above. First, cisplatin (75–80 mg/m2) was administered via the intravenous route on day 1 in the study by Hsieh and colleagues and in our study, but at 50 mg/m2 on days 1 and 8 in the study of Ngan et al. and 25 mg/m2 on days 1, 2 and 3 in the study of Wang et al. Second, the percentage of patients with a distant metastatic lesion was much higher in the study of Hsieh et al. and our study compared with that in the studies of Ngan et al. and Wang and coworkers (92.3–100% vs. 77.3–79.5%). Third, the mean number of cycles of the GC regimen was much higher in the study of Hsieh et al. and in our study compared with the studies of Ngan et al. and Wang and colleagues (4.6–6.6 vs. 3.6–4.8).
In 2003, Ong and colleagues [20] designed a scoring system for M-NPC and found that the negative prognostic factors were liver metastasis, lung metastasis, anemia, poor PS, distant metastasis at the initial diagnosis, and a disease-free interval of <6 months. In 2012, Jin and colleagues [21] found that the negative prognostic factors for patients with M-NPC were liver metastasis, high plasma level of Epstein–Barr virus-DNA (1×103 copies/mL), and receiving fewer than four cycles of first-line chemotherapy. In 2015, Hsieh and colleagues [16] demonstrated that, for patients with recurrent NPC or M-NPC receiving a GC regimen, the number of distant metastatic sites and liver metastasis were independent poor prognostic factors for OS, and that the number of metastatic sites was also a poor prognostic factor for PFS. Our results are consistent with those of Hsieh et al. In our study, Cox multivariate analyses revealed that the number of metastatic sites (P = .001) was an independent positive prognostic factor for PFS. A PFS benefit was observed for patients without liver metastases compared with patients with liver metastases in multivariate analyses, though this difference was not significant (P = .053). Hui et al. [22] conducted a retrospective trial and found that the presence of lung metastasis was an independent good prognostic factor for OS, but this finding was not replicated in our study or that of Hsieh et al. [16].
Conclusion
The GC+Endostar regimen was well-tolerated and, in general, effective chemotherapy in patients with M-NPC.
Materials and Methods
Inclusion Criteria and Enrollment
The inclusion criteria in this study consisted of patients: (1) with histologic confirmation of non-keratinizing carcinoma or undifferentiated NPC; (2) with radiologic confirmation of distant metastatic lesion(s); (3) with at least one radiologically measurable lesion according to the Response Evaluation Criteria In Solid Tumors (RECIST) v1.0 [23]; (4) of age 18–65 years; (5) with good performance status (Karnofsky performance status = 70–100) before treatment; (6) with normal renal (creatinine clearance ≥30 mL/min), cardiac and liver function (bilirubin level ≤1.5 mg/dL, levels of aspartate aminotransferase or alanine aminotransferase ≤2.5-times the upper limit of normal) and bone-marrow function (hemoglobin ≥10 g/dL, granulocytes ≥1500/μL, and platelet count ≥100,000/μL); (7) who received GC+Endostar as first-line chemotherapy for metastatic disease; (8) with complete clinical data.
Finally, 72 patients from Zhejiang Cancer Hospital (Hangzhou, China) were enrolled between July 2010 and November 2016. Ethical approval from the Institutional Review Board on Medical Ethics of Zhejiang Cancer Hospital was obtained before study commencement.
Treatment Plan
Cisplatin was added to 500 mL of physiologic (0.9%) saline and administered on day 1 (80 mg/m2, i.v.); gemcitabine was added to 100 mL of physiologic saline and administered on days 1 and 8 (1,000 mg/m2; i.v.). Endostar (15 mg/day dissolved in 500 mL of physiologic saline) was infused slowly (i.v.) from day 1 to day 14. This combination was repeated every 3 weeks. Dose modifications for GC+Endostar during chemotherapy were prescribed as noted in our previous study [11]. Chemotherapy was discontinued in case of disease progression, or patient refusal.
Evaluation of Treatment
Tumor response was evaluated every two cycles during chemotherapy and then every 3 months after completion of chemotherapy using RECIST v1.0. The response evaluation of the tumor to therapy was based on computed tomography or MRI findings. Short-term efficacy was assessed as a complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). A CR and PR were regarded to reflect the response to treatment. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 before each treatment cycle. Details of the assessment and monitoring of our patients are as noted in our previous study [11].
Statistical Analyses
PFS was defined as the duration from the first day of starting chemotherapy to disease progression (newly occurring metastatic lesion, recurrence or expansion of the primary lesion) by radiologic confirmation. Overall survival (OS) was calculated from the first day of starting chemotherapy to death from cancer or treatment-related toxicity. Patients who were alive until the last follow-up were recorded as ‘censored’.
Statistical analyses were undertaken using SPSS v16.0 (IBM, Armonk, NY, USA). Survival was analyzed using the Kaplan–Meier method and compared using the log-rank test. The difference in the frequency of each group in an individual category was analyzed by the chi-square test. All P-values were two-tailed and considered significant if P < .05.
Author Contributions
Ting Jin, Feng Jiang, Qi-Feng Jin, Yong-Feng Piao carried out the cases collection, Ting Jin and Feng Jiang analyzed results. Xiao-Zhong Chen conceived of the study, participated in its design and coordination and helped to draft the manuscript.
Acknowledgments
Acknowledgments
We thank Dr. Chan-Juan Tao, Dr. Yong-Hong Hua, Dr. Qiao-Ying Hu, and Dr. Fu-Jun Hu for their help with collecting the data.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the Zhejiang Medical and Health Science and Technology Platform Project [Grant No. 2017RC016]; the National Natural Science Foundation of China [Grant No. 81672971]; and the Excellent Talents Project of Zhejiang Cancer Hospital, P. R. China [Grant No. 2013 to T.J.].
References
- 1.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng WT, Lee MC, Chang AT, Chan OS, Chan LL, Cheung FY, Hung WM, Chan CC, Lee AW. The impact of dosimetric inadequacy on treatment outcome of nasopharyngeal carcinoma with IMRT. Oral Oncol. 2014;50(5):506–512. doi: 10.1016/j.oraloncology.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J, Tan TW, Fong KW, Chua ET, Wee JT. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys. 2009;75(5):1481–1486. doi: 10.1016/j.ijrobp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Q, Zhu X, Li L, Qu S, Liang Z, Zeng F, Pan X. IMRT combined with concurrent chemotherapy plus adjuvant chemotherapy versus IMRT combined with concurrent chemotherapy alone in patients with nasopharyngeal carcinoma. Oncotarget. 2017;8(24):39683–39694. doi: 10.18632/oncotarget.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, Deng X, Huang S, Lin C, Lu T. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103(1):22–31. doi: 10.1002/cncr.20768. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Ma BB, Ng WT, Chan AT. Managemen t of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, Peng P, Wu X, Lin Q, Xi X. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 9.Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM, Chua ET, Tan T, Khoo-Tan HS, Yang TL. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol. 1999;10(2):235–237. doi: 10.1023/a:1008390929826. [DOI] [PubMed] [Google Scholar]
- 10.Chua DT, Yiu HH, Seetalarom K, Ng AW, Kurnianda J, Shotelersuk K, Krishnan G, Hong RL, Yang MH, Wang CH. Phase II trial of capecitabine plus cisplatin as first-line therapy in patients with metastatic nasopharyngeal cancer. Head Neck. 2012;34:1225–1230. doi: 10.1002/hed.21884. [DOI] [PubMed] [Google Scholar]
- 11.Jin T, Li B, Chen XZ. A phase II trial of Endostar combined with emcitabine and cisplatin chemotherapy in patients with metastatic nasopharyngeal carcinoma ( NCT01612286) Oncol Res. 2013;21:317–323. doi: 10.3727/096504014X13983417587401. [DOI] [PubMed] [Google Scholar]
- 12.Leong SS, Wee J, Tay MH, Toh CK, Tan SB, Thng CH, Foo KF, Lim WT, Tan T, Tan EH. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma. Cancer. 2005;103:569–575. doi: 10.1002/cncr.20804. [DOI] [PubMed] [Google Scholar]
- 13.Ngan RK, Yiu HH, Lau WH, Yau S, Cheung FY, Chan TM, Kwok CH, Chiu CY, Au SK, Foo W. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13:1252–1258. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li J, Hong X, Tang W, Hu X, Wang B, Guo Y. Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol. 2008;44:464–470. doi: 10.1016/j.oraloncology.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Ma BB, Hui EP, Wong SC, Tung SY, Yuen KK, King A, Chan SL, Leung SF, Kam MK, Yu BK. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma-correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol. 2009;20:1854–1859. doi: 10.1093/annonc/mdp065. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh JC, Hsu CL, Ng SH, Wang CH, Lee KD, Lu CH, Chang YF, Hsieh RK, Yeh KH, Hsiao CH. Gemcitabine plus cisplatin for patients with recurrent or metastatic nasopharyngeal carcinoma in Taiwan: a multicenter prospective phase II trial. Jpn J Clin Oncol. 2015;45:819–827. doi: 10.1093/jjco/hyv083. [DOI] [PubMed] [Google Scholar]
- 17.Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 1850;2015:2422–2438. doi: 10.1016/j.bbagen.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, Chen Y, Guo QL. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 19.Guan Y, Li A, Xiao W, Liu S, Chen B, Lu T, Zhao C, Han F. The efficacy and safety of Endostar combined with chemoradiotherapy for patients with advanced, locally recurrent nasopharyngeal carcinoma. Oncotarget. 2015;6:33926–33934. doi: 10.18632/oncotarget.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, Tan T, Tan EH. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–1541. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y, Cai YC, Cao Y, Cai XY, Tan YT, Shi YX, Jiang WQ. Radiofrequency ablation combined with systemic chemotherapy in nasopharyngeal carcinoma liver metastases improves response to treatment and survival outcomes. J Surg Oncol. 2012;106:322–326. doi: 10.1002/jso.23034. [DOI] [PubMed] [Google Scholar]
- 22.Hui EP, Leung SF, Au JS, Zee B, Tung S, Chua D, Sze WM, Law CK, Leung TW, Chan AT. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]


