Fig. 4.
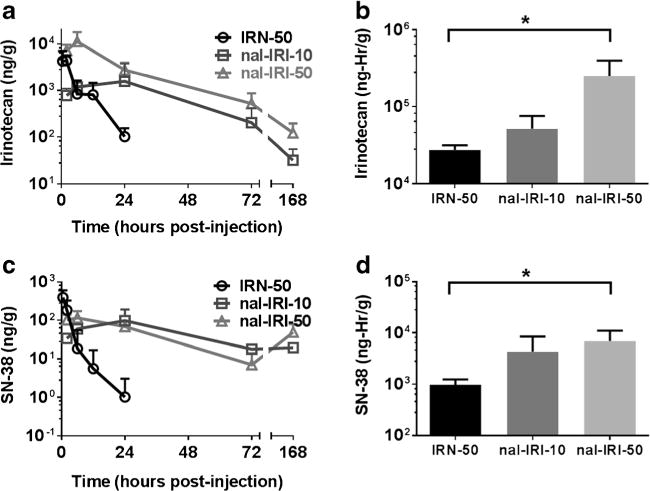
(a and c) Brain tumor concentration-time profiles of irinotecan and active metabolite SN-38 after IV bolus administration of IRN-50, nal-IRI-10 and nal-IRI-50. Irinotecan and SN-38 nearly cleared from circulation within 24 hr from IRN-50, whereas in nal-IRI-10 and nal-IRI-50 formulations, we observed a prolonged exposure of both irinotecan and SN-38 until 168 hr. (b and d) Brain tumor exposure of irinotecan and SN-38 expressed by area under the curve (AUC) after IV bolus administration of IRN-50, nal-IRI-10 and nal-IRI-50. Both Irinotecan and SN-38 AUCs for nal-IRI-50 were significantly higher than that of IRN-50 (p<0.05). Data represents mean ± SD for n=4 animals per time point.
