Abstract
Melanoma patients with additional positive lymph nodes in the completion lymph node dissection (CLND) following a positive sentinel lymph node (SLN) biopsy would have a poorer prognosis than patients with no additional positive lymph nodes. We hypothesize that the progression of disease from the SLN to the non-SLN compartment is orderly and is associated with the worsening of the disease status. Thus, the SLN and non-SLN compartments are biologically different in that cancer cells, in general, arrive in the SLN compartment before spreading to the non-SLN compartment. To validate this concept, we used a large cohort of melanoma patients from our prospective SLN database in an academic tertiary medical center. Adult cutaneous melanoma patients (n = 291) undergoing CLND after a positive SLN biopsy from 1994 to 2009 were analyzed. Comparison of 5-year disease-free survival and 5-year overall survival between positive (n = 66) and negative (n = 225) CLND groups was made. The 5-year disease-free survival rates were 55% (95% CI 49–62%) for patients with no additional LN on CLND versus 14% (95% CI 8–26%) in patients with positive LN on CLND (p < 0.0001, log-rank test). The median disease-free survival time was 7.4 years with negative CLND (95% CI 4.4–15+ years) and 1.2 years with positive CLND (95% CI 1.0–1.8 years). The 5-year overall survival rates were 67% (95% CI 61–74%) for negative CLND versus 38% (95% CI 28–52%) for positive CLND (p < 0.0001, log-rank test). The median overall survival time was 12.1 years for negative CLND (95% CI 9.3–15+ years) and 2.5 years for positive CLND (95% CI 2.2–5.7 years). This study shows that CLND status is a significant prognostic factor for patients with positive SLNs undergoing CLND. Also, it suggests an orderly progression of metastasis from the SLN to the non-SLN compartment. Thus, the SLN in the regional nodal basin draining the primary melanoma may serve as an important gateway for metastasis to the non-SLN compartment and beyond to the systemic sites.
Keywords: Cutaneous melanoma, Sentinel lymph node biopsy, Complete lymph node dissection, Overall survival, Disease free survival
Introduction
Since the introduction of the sentinel lymph node (SLN) biopsy procedure by Morton in 1992 [1], it has become the standard of treatment for melanoma patients with Breslow thickness equals or greater than 1.0 mm (stages IB through IIC) as recommended by current National Comprehensive Cancer Network (NCCN) guidelines [2]. Recent evidence shows that the indication may be expanded to less than 1 mm especially if the primary melanoma is associated with higher risk features such as ulceration and mitosis [3]. In general, SLN mapping and selective SLN dissection have become standard staging procedure for patients with primary melanoma with clinically negative nodal basins.
Despite the fact that patients with additional positive lymph nodes from the complete lymph node dissection (CLND) would have a worse outcome [4–15], there has been controversy surrounding the performance of immediate CLND in patients with tumor positive SLNs. The primary concern about CLND is related to morbidity related to the procedure, which consists primarily of lymphedema, as high as 25% with axillary dissection and 48% with inguinal dissection [16], seroma and wound infections. Several studies have reported much lower complication rates for SLN biopsy, ranging from 5 to 14% versus those for CLND, between 23 and 66% [17–22].
Since there is only a reported 20% chance of having non-sentinel lymph node (NSLN) positivity [23], after a positive SLN biopsy, there are some concerns about morbidity in patients who wouldn't otherwise benefit from a negative CLND procedure. Thus, some authors suggest only observation and CLND to be performed only in patients with clinical recurrence [24, 25]. One major reason why the outcome of patients with a positive SLN biopsy is not the same is probably because of the enormous heterogeneity of SLN micrometastasis relating to the size and location of micrometastasis within the SLN [6, 7, 9, 26–28], and anatomic locations of SLNs [10].
Recently some groups have tried to develop risk assessment scores to correctly identify patients with high risk of NSLN involvement and who would benefit the most from CLND [4–8], with an attempt to identify the subgroup of patients who would benefit from a CLND. Although certain factors are found to be statistically significant, none of these factors are specific enough to predict the result of the CLND for each patient to be useful to recommend each patient the need for a CLND. In a separate population-based study from the Surveillance Epidemiology and End Results Program registry, the likelihood of CLND is significantly associated with age, gender and geographic area [29].
We have followed our patients with a positive SLN biopsy undergoing CLND at least over 5 years. The overall survival and disease free survival between patients with positive and negative CLND status following a positive SLN biopsy have been analyzed and compared. The goal of this study is to demonstrate the clinical outcome of these 2 groups of patients and to evaluate the prognostic significance of CLND.
Materials and methods
The analyses were based on reviewing a prospective database of 2079 cutaneous melanoma patients undergoing SLN dissection, collected from a tertiary care referral center from 1993 to 2011. Out of 2109 of primary melanoma patients undergoing SLN biopsy, 353 patients (16.7%) had positive SLNs; of these, there were 293 in which CLND had been performed. After excluding 2 patients under age 18, a total of 291 adult cases is used for the analysis of this paper (Fig. 1). A positive node was considered when pathologic review of the specimen yielded a positive result on Hematoxilin and Eosin stain for melanoma cells, most of the times corroborated with inmunohistochemical staining (Melan-A, S100 and HMB-45). A sentinel node was considered as the one with the highest radioactive tracer activity, and every subsequent node which was at least higher than 10% of the highest count [30, 31].
Fig. 1.
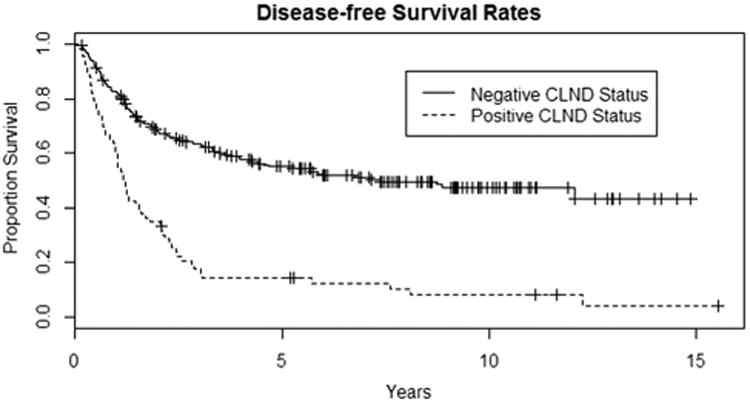
Kaplan–Meier curves to show that the negative-CLND group has a much better disease-free survival than the positive-CLND group (p<0.0001)
Statistical calculations
For the purpose of this study we separated the group with a positive SLN from the rest, and then split the group between patients with positive and negative CLND results. Baseline difference between two groups were evaluated. Five-year disease-free survival was calculated from the date of SLN dissection to the first recurrence of melanoma (either local, regional or distant). Five-year overall survival was calculated as the interval between SLN dissection and death due to any cause.
Patients without such an event were censored at the date of the last follow-up visit or the date the patient was last known to be alive. Survival curves and estimated 5-year survival rates with 95% confidence interval (95% CI) between groups were generated using Kaplan–Meier non-parametric method and compared by log-rank test.
To determine if the difference in survival was due to possible effects of the baseline group difference, Cox regression model was performed to estimate the relative hazards ratio by group after adjustment for the baseline covariates that were significantly different at baseline.
P values of less than 0.05 were considered statistically significant.
Results
Of 291 patients in our analysis population, 182 were males (62.5%) and 109 were females (37.5%) with a mean age of 52.0 years (range 18–87). The anatomic locations involved were 34 head and neck (H&N) cases, 128 trunk, 43 upper extremities, and 86 lower extremities.
Table 1 compares differences between CLND positive and negative patients. As shown in this table, there were no significance difference between the two patient groups in age, ethnicity, tumor regression, or mitotic rate. However, gender, Breslow thickness, Clark level, ulceration, lymphovascular invasion, and microsatellitosis were significantly different.
Table 1. Clinical and histologic features comparing CLND positive and negative patients.
| CLND− (N = 225) | CLND+ (N = 66) | Total (N = 291) | p value | |
|---|---|---|---|---|
| Age | 0.9284 | |||
| Mean | 52.1 | 51.9 | 52.0 | |
| SD | 15.7 | 15.9 | 15.7 | |
| Range | 18.1–87.2 | 18.3–82.4 | 18.1–87.2 | |
| Age group | 0.9371 | |||
| <60 | 155 (69%) | 47 (71%) | 202 (69%) | |
| 60–75 | 48 (21%) | 13 (20%) | 61 (21%) | |
| 75+ | 22 (10%) | 6 (9%) | 28 (10%) | |
| Gender | 0.0011 | |||
| Male | 152 (68%) | 30 (45%) | 182 (63%) | |
| Female | 73 (32%) | 36 (55%) | 109 (37%) | |
| Ethnicity | 0.1515 | |||
| White | 190 (84%) | 53 (80%) | 243 (84%) | |
| African-American | 0 (0%) | 1 (2%) | 1 (0.3%) | |
| Asian | 3 (1%) | 0 (0%) | 3 (1%) | |
| Other | 1 (0.4%) | 1 (2%) | 2 (0.7%) | |
| Breslow thickness (mm) | <0.0001 | |||
| Mean | 2.93 | 4.75 | 3.32 | |
| SD | 2.35 | 3.15 | 2.64 | |
| Range | 0.5–9.6 | 0.7–6.0 | 0.5–9.6 | |
| Breslow thickness group | 0.0001 | |||
| <2.00 | 69 (31%) | 5 (8%) | 74 (25%) | |
| 2.00–2.99 | 41 (18%) | 10 (15%) | 51 (18%) | |
| 3.00–3.99 | 17 (8%) | 7 (11%) | 24 (8%) | |
| ≥4.00 | 37 (16%) | 23 (35%) | 60 (21%) | |
| Clark level group | 0.0429 | |||
| II/III | 51 (23%) | 8 (12%) | 59 (20%) | |
| IV/V | 149 (66%) | 53 (80%) | 202 (69%) | |
| Ulceration | 0.0002 | |||
| Present | 71 (32%) | 39 (59%) | 110 (38%) | |
| Absent | 110 (49%) | 19 (28%) | 129 (44%) | |
| Lymphovascular invasion | 0.0052 | |||
| Present | 34 (15%) | 22 (33%) | 56 (19%) | |
| Absent | 102 (45%) | 25 (38%) | 127 (44%) | |
| Tumor regression | 0.4651 | |||
| Present | 16 (7%) | 3 (5%) | 19 (7%) | |
| Absent | 126 (56%) | 38 (58%) | 164 (56%) | |
| Microsatellitosis | 0.0369 | |||
| Present | 14 (6%) | 11 (17%) | 25 (9%) | |
| Absent | 111 (49%) | 35 (53%) | 146 (50%) | |
| Mitotic rate | 0.7916 | |||
| <1.0 | 3 (1%) | 1 (2%) | 4 (1%) | |
| ≥1.0 | 151 (67%) | 37 (56%) | 188 (65%) |
Not all patients had full complement of data points
Site specific recurrence
Out of the 142 patients with a recurrence recorded, their first recurrence, 28 were local (19.7%), 26 regional (18.3%) which account for in-transit or satellite nodes, 10 were in a regional basin without previous dissection (7.0%), 15 in a regional basin with a previous dissection (10.6%), 59 in a distant site (41.5%), most of them in the lung, and 4 in an unspecified location (2.8%).
Five year disease-free survival and overall survival
Two hundred twenty-five (225) patients had negative CLND and 66 had positive CLND (22.7%). The 5-year disease-free survival rates were 55% (95% CI 49–62%) for patients with no additional LN on CLND versus 14% (95% CI 8–26%) in patients with positive LN on CLND (p < 0.0001, log-rank test). The median disease-free survival time was 7.4 years with CLND negative (95% CI 4.4–15+ years) and 1.2 years with CLND positive (95% CI 1.0–1.8 years) (Fig. 1).
The 5-year overall survival rates were 67% (95% CI 61–74%) for negative CLND versus 38% (95% CI 28–52%) for positive CLND (p < 0.0001, log-rank test). The median overall survival time was 12.1 years for CLND negative (95% CI 9.3–15+ years) and 2.5 years for CLND positive (95% CI 2.2–5.7 years) (Fig. 2).
Fig. 2.
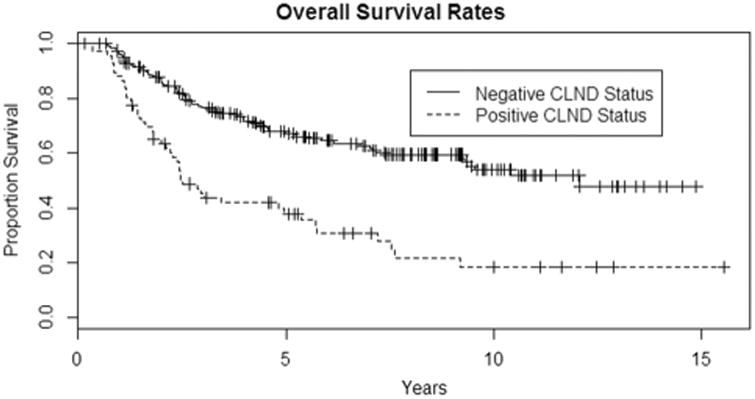
Kaplan–Meier curves to show that the negative-CLND group has a much better overall survival than the positive-CLND group (p = 0.0001)
A Cox proportional regression analysis was also calculated to estimate the relative hazards ratio between CLND group after adjustment of the difference in baseline covariates of gender, Breslow index, Clark's level, ulceration, lymphovascular invasion, and microsateliitosis that were significantly different in Table 1. The estimated hazards ratio for disease-free survival by CLND positive group is 2.40 with a 95% confidence interval (1.49, 3.86) (p = 0.0003, log-rank test). Similarly, the hazards ratio for overall survival is 2.15 with a 95% confidence interval (1.32, 3.50) (p = 0.0022, log-rank test).
Discussion
The concept that cancer cells from the microenvironment of the primary site would spread to the SLN(s) in the regional nodal basin [1] has been convincingly validated in melanoma [11] and breast cancer [32]. In the pre-SLN era, regional lymph node metastasis has been considered as an indicator rather than governor regarding cancer progression [33]. This concept may be challenged in the SLN era if micrometastasis in the SLN is merely an indicator of systemic disease or if it is being incubated within the SLN to become more aggressive to spread to the systemic sites. In most cases for melanoma [11] and breast cancer [32], perhaps, it is a spectrum of event from the initial arrival of cancer cells in the SLN, which functions like an incubator to allow the cancer cells to proliferate and then spread to the non-SLN compartment and the systemic sites. The tumor microenvironment of the SLN serves as a Darwinian selection force [34] as to allow the emergence of the “fittest clone” to spread to the distant sites. The SLN provides us with a unique opportunity to study the progression of cancer cells in the nodal tissue from micrometastasis to macrometastasis and beyond to the distant sites.
Numerous studies have shown that melanoma patients with a positive CLND following a positive SLN biopsy have a worse prognosis than those with a negative dissection [4–15]. One of the largest series by Rutkowski et al. [15] with 473 melanoma patients undergoing CLND following a positive SLN biopsy from a cohort of 1764 consecutive melanoma patients showed that 8 year overall survival rate in the entire group was 73.5%, 80% without SLN metastases and 50% in the SLN+ group (p < 0.001). Leung et al. [35] have shown that non-SLN positivity is one of the most significatnt prognostic factors in Stage III melanoma patients with micrometastasis in SLN(s). Thus, they have proposed that non-SLN positivity should be used as an AJCC subclassification of nodal stage. An attempt was made by Roka et al. to identify the subgroup of patients with a positive NSLN status using clinicopathological features, but they were not able to find a difference between SLN-only metastasis and NSLN groups [36].
In general, solid cancer is a progressive disease, consistent with the spectrum theory of cancer progression [37], from the microenvironment of the primary site to the SLN gateway and beyond to the distant sites by way of the bone marrow and peripheral blood [38], perhaps in about 20% of the time. Thus, it is important to follow the SLN-negative patients for distant metastasis without local and/or regional recurrences as the cancer cells in these patients may have potentially spread from the primary site by hematogenous routes to distant sites.
The recently completed American College of Surgeons Oncology Group Z0011 trial, to examine whether CLND is necessary in early breast cancer patients with clinically non-palpable, but histopathology positive SLNs has shown that for such patients with no clinical palpable adenopathy but a positive SLN biopsy may not need an axillary lymph node dissection [39]. Thus, these findings suggest that when the SLN contains micrometastasis, the disease is probably limited to the SLN without further progression. The ultimate goal for cancer staging is to develop molecular biomarkers for cancer cells during their progression from the primary site to the regional nodes and beyond to the systemic sites [38, 40] so that therapy can be directed more precisely with personalized approaches based on molecular taxonomy.
Since micrometastasis is detected only occasionally in NSLNs from the CLND specimens, it has been argued that CLND is not required after a positive SLN biopsy. In general, each NSLN is examined by one hemotoxylin-eosin section. Thus, the lack of more extensive immunohistochemical staining of multiple sections for NLSNs has been assumed to result in low yield of positive NLSNs. Holt-kamp et al. have launched a study to examine a total of 21 tumor-negative CLND specimens from 20 patients with SLN micrometasis less than 0.1 mm in diameter using 5 additional sections stained with/for H&E, S-100, HMB45, Melan-A and H&E. One out of 20 patients was found to have additional micrometastasis in the NSLN. The authors admit that the risk of NSLN involvement with micrometastasis is low in this selective group of patients with SLN micrometastasis of only less than 0.1 mm, they maintain that nodal resection may be the safest alternative for patients with SLN micrometastasis [41]. No such detailed studies with multiple sections for NSLNs for patients with SLN micrometastasis larger than 0.1 mm. Perhaps, the NSLN positivity rate may go up with increasing size of SLN micrometastasis.
Recently, the German Dermatologic Oncology Group (DeCOG)-SLT trial has completed a phase III study which randomized 483 patients with a positive SLN biopsy to either CLND or nodal observation. The results showed that CLND following a positive SLN biopsy did not pro-long distant metastasis-free, recurrence-free survival or improved overall survival in positive SLN patients when compared with the cohort having nodal observation only with a median follow-up of 35 months. Most of the patients in this study have a low tumor burden in SLNs not exceeding a diameter of 1 mm (311; 66%). Comparing observation versus CLND in patients with melanoma with single cells or micrometastases, the CLND group showed better regional nodal basin control. On the basis of the findings from the DeCOG-SLT trial, CLND may not be indicated in melanoma patients with micrometastases, at least those with single cells or mircometastases, 1 mm in size or smaller [42]. The DeCOG-SLT trial data may be criticized for lack of large number of enrolled patients and its median follow-up period is relatively short. The long-awaited MSLT II clinical trial results have just been published [43]. Although immediate CLND decreased the regional disease recurrence rate and provided useful prognostic information, it did not increase melanoma-specific survival among patients with sentinel-node metastases. One critique is that the SLN tumor burden was quite low with a median diamter of about 0.6 mm. In fact, about three quarters of the SLN-positive patients had a negative CLND, thus, potentially diluting the therapeutic effect. In addition to SLN status, other features such as primary melanoma sites, heterogeneity of micrometastases and melanoma thickness may influence recurrence and survival. Data from EORTC 1208 MiniTub registration study may shed additional light to these issues (EORTC protocol 1208-MG, NCT01942603).
In this study, we have shown that about 20% of the adult patients with a positive SLN biopsy undergoing CLND have additional lymph nodes in the NSLN compartment, which carry a worse prognosis. The orderly progression of metastasis from the SLN to the NSLN compartment [44] has been firmly established in this study. Thus, the SLN compartment in the regional nodal basin draining the primary melanoma may serve as an important gateway for metastasis to the non-SLN compartment and beyond to the systemic sites.
Acknowledgments
Dr. Cleaver is supported by “E.A.Dickson Emeritus Professorship” and by a gift from the Tumori Foundation courtesy of Dr. Devron Char, CPMC.
References
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 2.Coit DG, Thompson JA, Andtbacka R, et al. Melanoma, version 4.2014. J Natl Compr Canc Netw. 2014;12:621–629. doi: 10.6004/jnccn.2014.0066. [DOI] [PubMed] [Google Scholar]
- 3.Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31:4387–4393. doi: 10.1200/JCO.2013.50.1114. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Essner R, Torisu-Itakura H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677–3684. doi: 10.1200/JCO.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Murali R, Desilva C, Thompson JF, et al. Non-Sentinel Node Risk Score (N-SNORE): a scoring system for accurately stratifying risk of non-sentinel node positivity in patients with cutaneous melanoma with positive sentinel lymph nodes. J Clin Oncol. 2010;28:4441–4449. doi: 10.1200/JCO.2010.30.9567. [DOI] [PubMed] [Google Scholar]
- 6.van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol. 2011;29:2206–2214. doi: 10.1200/JCO.2010.31.6760. [DOI] [PubMed] [Google Scholar]
- 7.van Akkooi AC, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248:949–955. doi: 10.1097/SLA.0b013e31818fefe0. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraja V, Eslick GD. Is complete lymph node dissection after a positive sentinel lymph node biopsy for cutaneous melanoma always necessary? A meta-analysis. Eur J Surg Oncol. 2013;39:669–680. doi: 10.1016/j.ejso.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Baehner FL, Li R, Jenkins T, et al. The impact of primary melanoma thickness and microscopic tumor burden in sentinel lymph nodes on melanoma patient survival. Ann Surg Oncol. 2012;19:1034–1042. doi: 10.1245/s10434-011-2095-3. [DOI] [PubMed] [Google Scholar]
- 10.Han D, Thomas DC, Zager JS, et al. Clinical utilities and biological characteristics of melanoma sentinel lymph nodes. World J Clin Oncol. 2016;7:174–188. doi: 10.5306/wjco.v7.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton DL. Overview and update of the phase III Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) in melanoma. Clin Exp Metastasis. 2012;29:699–706. doi: 10.1007/s10585-012-9503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunte C, Geimer T, Baumert J, et al. Prognostic factors associated with sentinel lymph node positivity and effect of sentinel status on survival: an analysis of 1049 patients with cutaneous melanoma. Melanoma Res. 2010;20:330–337. doi: 10.1097/CMR.0b013e32833ba9ff. [DOI] [PubMed] [Google Scholar]
- 13.Brown RE, Ross MI, Edwards MJ, et al. The prognostic significance of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol. 2010;17:3330–3335. doi: 10.1245/s10434-010-1208-8. [DOI] [PubMed] [Google Scholar]
- 14.Glumac N, Hocevar M, Zadnik V, et al. Sentinel lymph node micrometastasis may predict non-sentinel involvement in cutaneous melanoma patients. J Surg Oncol. 2008;98:46–48. doi: 10.1002/jso.21066. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski P, Szydlowski K, Nowecki ZI, et al. The long-term results and prognostic significance of cutaneous melanoma surgery using sentinel node biopsy with triple technique. World J Surg Oncol. 2015;13:299. doi: 10.1186/s12957-015-0701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ul-Mulk J, Holmich LR. Lymph node dissection in patients with malignant melanoma is associated with high risk of morbidity. Dan Med J. 2012;59:A4441. [PubMed] [Google Scholar]
- 17.Satzger I, Meier A, Zapf A, et al. Is there a therapeutic benefit of complete lymph node dissection in melanoma patients with low tumor burden in the sentinel node? Melanoma Res. 2014;24:454–461. doi: 10.1097/CMR.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 18.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–311. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guggenheim MM, Hug U, Jung FJ, et al. Morbidity and recurrence after completion lymph node dissection following sentinel lymph node biopsy in cutaneous malignant melanoma. Ann Surg. 2008;247:687–693. doi: 10.1097/SLA.0b013e318161312a. [DOI] [PubMed] [Google Scholar]
- 20.Kretschmer L, Thoms KM, Peeters S, et al. Postoperative morbidity of lymph node excision for cutaneous melanoma-sentinel lymphonodectomy versus complete regional lymph node dissection. Melanoma Res. 2008;18:16–21. doi: 10.1097/CMR.0b013e3282f2017d. [DOI] [PubMed] [Google Scholar]
- 21.van Akkooi AC, Bouwhuis MG, van Geel AN, et al. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol. 2007;33:102–108. doi: 10.1016/j.ejso.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Holmes EC, Moseley HS, Morton DL, et al. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–490. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13:809–816. doi: 10.1245/ASO.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 25.van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma without immediate completion lymph node dissection. Br J Surg. 2012;99:1396–1405. doi: 10.1002/bjs.8878. [DOI] [PubMed] [Google Scholar]
- 26.van Akkooi AC, de Wilt JH, Verhoef C, et al. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17:1578–1585. doi: 10.1093/annonc/mdl176. [DOI] [PubMed] [Google Scholar]
- 27.van der Ploeg AP, van Akkooi AC, Haydu LE, et al. The prognostic significance of sentinel node tumour burden in melanoma patients: an international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer. 2014;50:111–120. doi: 10.1016/j.ejca.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Averbook BJ. Mitotic rate and sentinel lymph node tumor burden topography: integration into melanoma staging and stratification use in clinical trials. J Clin Oncol. 2011;29:2137–2141. doi: 10.1200/JCO.2010.34.1982. [DOI] [PubMed] [Google Scholar]
- 29.Mosquera C, Vora HS, Vohra N, et al. Population-based analysis of completion lymphadenectomy in intermediate-thickness melanoma. Ann Surg Oncol. 2017;24(1):127–134. doi: 10.1245/s10434-016-5460-4. [DOI] [PubMed] [Google Scholar]
- 30.Kroon HM, Lowe L, Wong S, et al. What is a sentinel node? Re-evaluating the 10% rule for sentinel lymph node biopsy in melanoma. J Surg Oncol. 2007;95:623–628. doi: 10.1002/jso.20729. [DOI] [PubMed] [Google Scholar]
- 31.Murphy AD, Britten A, Powell B. Hot or not? The 10% rule in sentinel lymph node biopsy for malignant melanoma revisited. J Plast Reconstr Aesthet Surg. 2014;67:316–319. doi: 10.1016/j.bjps.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–399. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cady B. Lymph node metastases. Indicators, but not governors of survival. Arch Surg. 1984;119:1067–1072. doi: 10.1001/archsurg.1984.01390210063014. [DOI] [PubMed] [Google Scholar]
- 34.Hellman S. Darwin's clinical relevance. Cancer. 1997;79:2275–2281. doi: 10.1002/(sici)1097-0142(19970615)79:12<2275::aid-cncr1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Leung AM, Morton DL, Ozao-Choy J, et al. Staging of regional lymph nodes in melanoma: a case for including nonsentinel lymph node positivity in the American Joint Committee on Cancer staging system. JAMA Surg. 2013;148:879–884. doi: 10.1001/jamasurg.2013.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roka F, Mastan P, Binder M, et al. Prediction of non-sentinel node status and outcome in sentinel node-positive melanoma patients. Eur J Surg Oncol. 2008;34:82–88. doi: 10.1016/j.ejso.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 38.Leong SP, Tseng WW. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: Clinical significance and biologic implications. CA Cancer J Clin. 2014;64:195–206. doi: 10.3322/caac.21217. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leong SP, Witte M. Biomarkers of cancer metastasis through the lymphovascular system: future perspectives. Clin Exp Metastasis. 2012;29(7):861–864. doi: 10.1007/s10585-012-9522-0. [DOI] [PubMed] [Google Scholar]
- 41.Holtkamp LH, Wang S, Wilmott JS, et al. Detailed pathological examination of completion node dissection specimens and outcome in melanoma patients with minimal (< 0.1 mm) sentinel lymph node metastases. Ann Surg Oncol. 2015;22:2972–2977. doi: 10.1245/s10434-015-4615-z. [DOI] [PubMed] [Google Scholar]
- 42.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–767. doi: 10.1016/S1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 43.Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, Wouters MWJM, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, Neves RI, Trocha SD, Wright F, Byrd DR, Matter M, Hsueh E, MacKenzie-Ross A, Johnson DB, Terheyden P, Berger AC, Huston TL, Wayne JD, Smithers BM, Neuman HB, Schneebaum S, Gershenwald JE, Ariyan CE, Desai DC, Jacobs L, McMasters KM, Gesierich A, Hersey P, Bines SD, Kane JM, Barth RJ, McKinnon G, Farma JM, Schultz E, Vidal-Sicart S, Hoefer RA, Lewis JM, Scheri R, Kelley MC, Nieweg OE, Noyes RD, Hoon DSB, Wang HJ, Elashoff DA, Elashoff RM. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leong SP. Compartmentalizing sentinel lymph nodes and nonsentinel lymph nodes in regional lymph nodes draining the primary melanoma. JAMA Surg. 2013;148:884–885. doi: 10.1001/jamasurg.2013.3057. [DOI] [PubMed] [Google Scholar]


