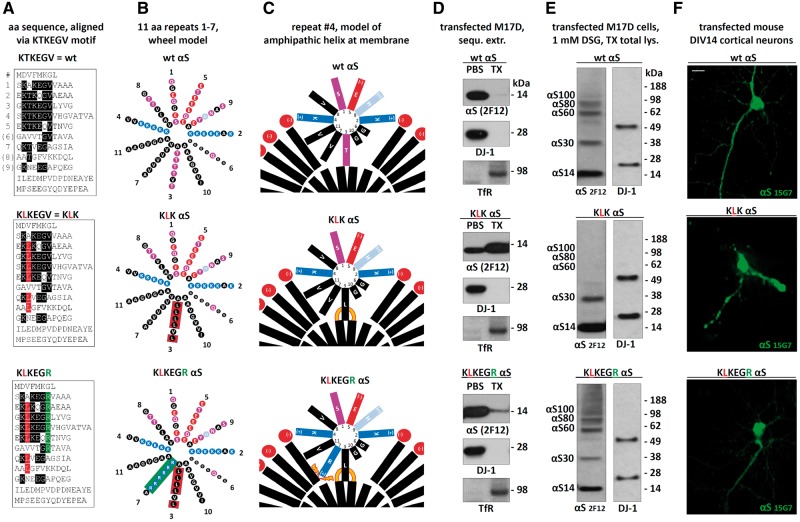
Figure 8.
Rescuing the negative effects of αS KLK by introducing charged residues into the hydrophobic half of the αS amphipathic helix. (A) Schematics of αS wt, KLK and KLKEGR by aligning aa sequences via the repeat consensus sequence KTKEGV. (B) αS 11-residue repeats arranged in a helical wheel (a 3–11 helix: 3 turns over eleven residues). Blue indicates basic (light blue: histidine), red: acidic, purple: polar uncharged, and black: non-polar residues. (C) Schematic of αS repeat #4 embedded in the outer leaflet of a curved vesicle membrane (lipid head-groups in red, fatty acid ‘tails’ in black). Orange lines illustrate increased αS-membrane attraction, orange thunderbolts indicate increased αS-membrane repulsion. (D) WB for αS (mAb 2F12) in the PBS- or TX-100-soluble fractions of the indicated M17D transfectants. DJ-1 (cytosolic) and Transferrin receptor (membrane) served as controls; representative of three independent experiments. (E) wt, KLK and KLKEGR αS were transiently expressed in M17D cells for 48 h followed by in vivo cross-linking and WB; mAb 2F12 to αS; DJ-1 pAb as a control for equal loading and crosslinking. (F) Fluorescence microscopy of DIV14 mouse primary neurons transfected with the indicated untagged αS variants; human-specific αS mAb 15G7; scale bar, 20 μm; representative of three independent experiments.