Figure 1. Lysophosphatidylcholine (LysoPC) tips the balance away from sexual differentiation.
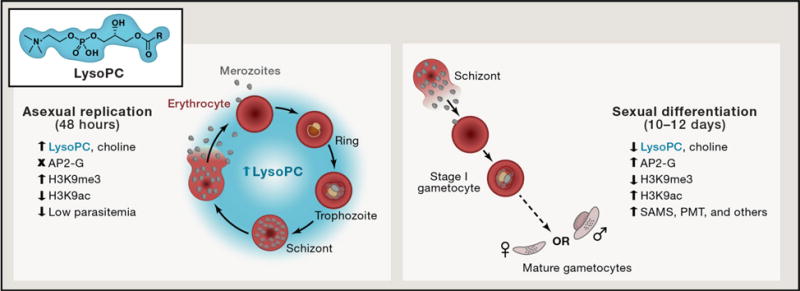
The balance between asexual parasite replication and sexual differentiation is explored in every 48-hour cycle of the human malaria parasite Plasmodium falciparum’s developmental cycle. Brancucci and Gerdt et al. have identified lysophosphatidylcholine (LysoPC) as an important molecule whose presence (at low parasitemia) represses conversion to the sexual gametocyte form required for transmission to the mosquito host. LysoPC is a host-derived lipid that gets metabolized by the parasite into critical membrane phospholipids. Decreased levels of LysoPC result in transcriptional activation of key enzymes required to replenish phospholipid stores via S-adenosylmethioine (SAM) – mediated reactions including S-adenosylmethioine synthetase (SAMS) and phosphoethanolamine methyltransferase (PMT). This diversion of SAM suggests a potential interplay of metabolic adjustments that likely result in a decrease in H3K9me3 and a concomitant de-repression of the gene encoding the critical AP2-G transcription factor (Kafsack et al., 2014) required to drive gametocyte commitment and differentiation.
