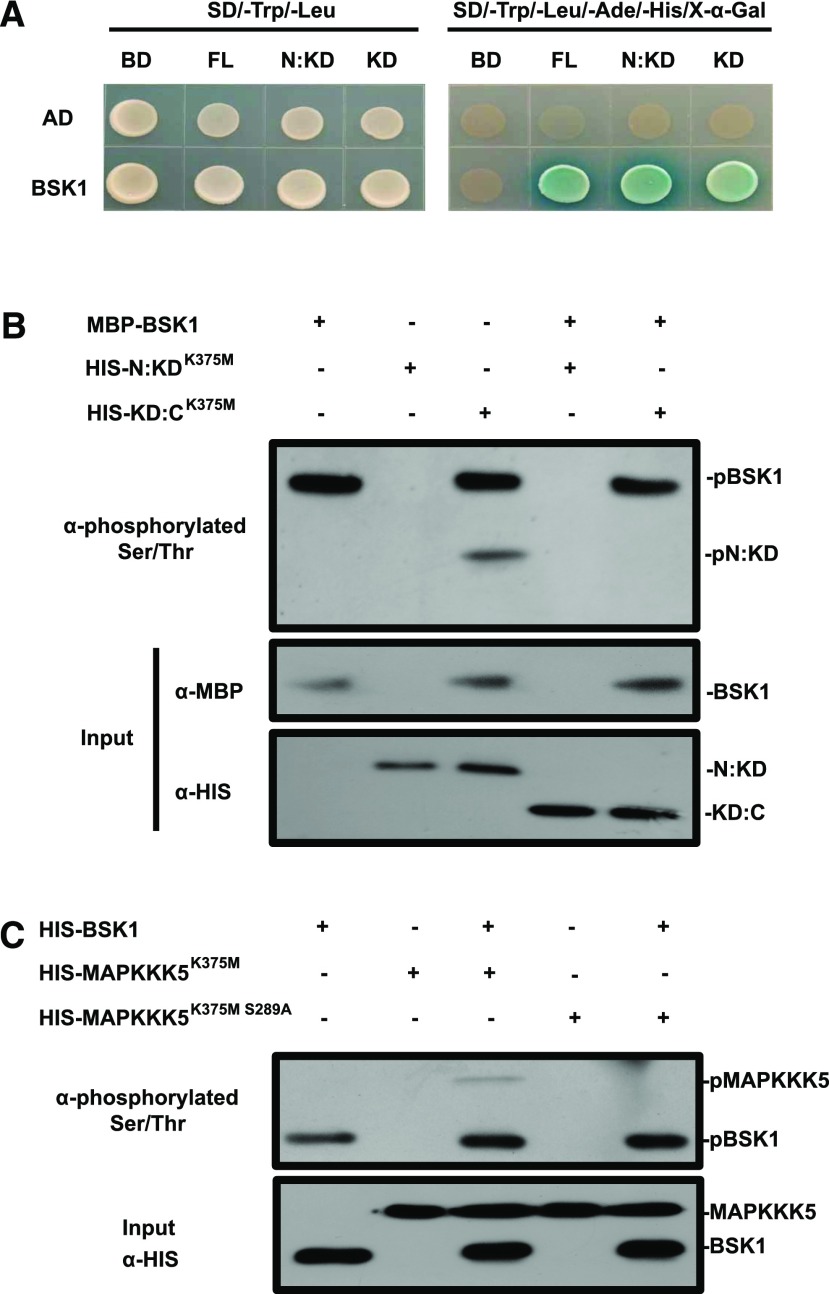
Figure 3.
BSK1 phosphorylates the N terminus of MAPKKK5 in vitro. A, The interaction of the truncated versions of MAPKKK5 with BSK1 was examined in the yeast two-hybrid assay. Corresponding constructs were cotransformed into yeast strain AH109. A 10 μL suspension of each pair was dropped on the indicated SD medium. Pictures were taken 3 d after incubation. B, BSK1 phosphorylates the N terminus of MAPKKK5 in vitro. The in vitro phosphorylation was revealed by an antibody that could specifically detect phosphorylated Ser and Thr. The phosphorylated proteins were detected by an immunoblot assay with the indicated antibodies. The phosphorylated N:KD fragment of MAPKKK5 and BSK1 was labeled. C, BSK1 phosphorylates the Ser-289 of MAPKKK5 in vitro. The phosphorylated proteins were detected by an immunoblot assay with the antibodies that could specifically detect phosphorylated Ser and Thr. The phosphorylated MAPKKK5 and BSK1 were labeled. AD, pGADT7; BD, pGBKT7; KD, kinase domain; KD:C, MAPKKK5 lacking the N terminus of 345 amino acids; N:KD, MAPKKK5 lacking the C terminus of 109 amino acids; NT, N-terminal domain.