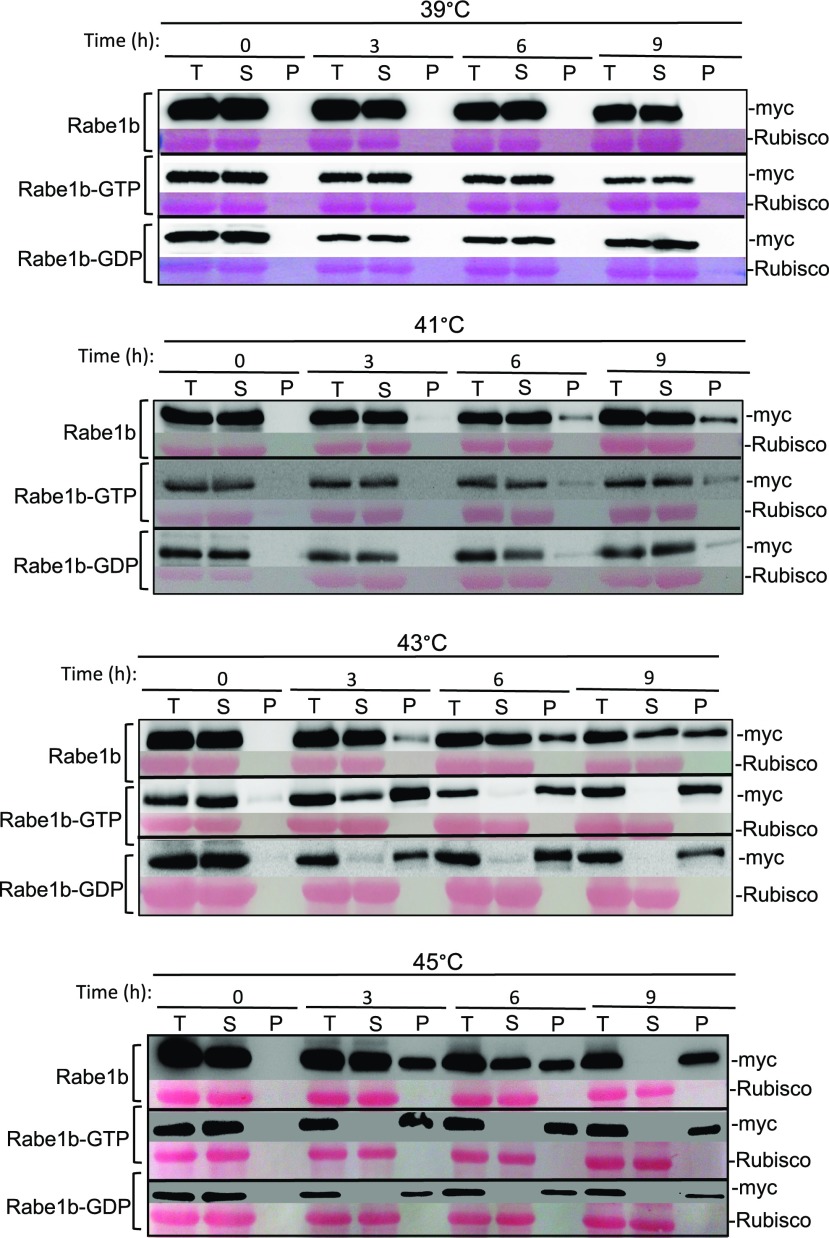
Figure 3.
Heat-induced rapid aggregation of Arabidopsis plastid EF-Tu in vivo. Transgenic AtRabe1b-OE, AtRabe1b-GTP-OE, and AtRabe1b-GDP-OE Arabidopsis plants were treated at the indicated temperatures for the indicated hours. Total proteins (T) were extracted from treated transgenic plants and separated by low-speed centrifugation into soluble proteins in the supernatants (S) and insoluble proteins in the pellets (P). Myc-tagged AtRabe1b, AtRabe1b-GTP, and AtRabe1b-GDP proteins in the total, soluble, and insoluble fractions were analyzed by immunoblotting. Rubisco large subunit proteins stained with Ponceau S are shown as the loading control.