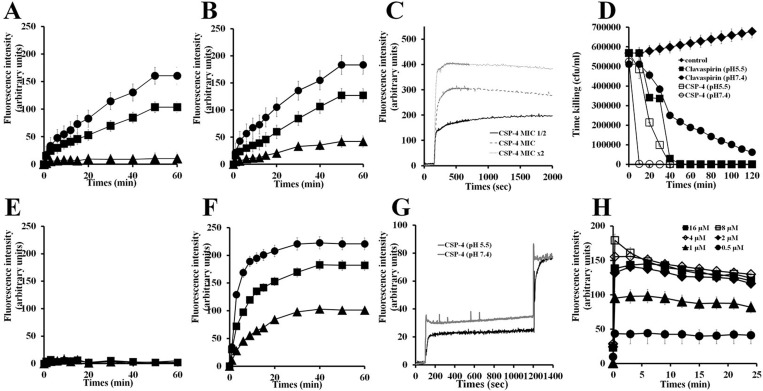
Figure 6. Mechanism of action of CSP and its analogue peptides against E. coli and S. aureus.
E. coli (2 × 107 cfu/mL) were incubated with 1 μM SYTOX-Green for 15 min. CSP (A, E) and CSP-4 (B, F) were added when basal fluorescence reached a constant value in 10 mM sodium phosphate buffer at pH 5.5 (A, B) or pH 7.4 (E, F). (C) Fluorescence was monitored at the indicated times (Ex. 485 nm and Em. 520 nm). Hyperpolarization was induced by incubation for 5 min on ice followed by incubation at 37° C with disC3-5 (0.5 mM) for 10 min. (D) CSP-4 killing kinetics. E. coli cells in not a growth phase were adjusted to OD600 0.06, CSP-4 was then added at its MIC or 2× MIC, and changes in fluorescence were monitored continuously (Ex. 622 nm and Em. 670 nm) and plotted as arbitrary units. (G) CSP-4-induced release of FD-FITC-dextran from LUV liposomes (PE/PG, 7/3, w/w). Effect of pH on CSP-4-induced release of FD-FITC-dextran from LUV liposomes (PE/PG, 7/3, w/w). CSP-4 was added at the MIC to LUVs entrapping FD-FITC-dextran (100 mg/mL) dye in HEPES buffer at pH 5.5 or pH 7.4. The dotted line shows the simultaneous determinations of bacterial viability after exposure of E. coli cell to the MIC or 2× MIC of CSP-4. (H) Effect of CSP-4 on S. aureus membrane permeability. S. aureus cells (2 × 107 cfu/mL) were incubated for 15 min with 1 μM SYTOX-Green. CSP-4 was added when basal fluorescence reached a constant value in 10 mM sodium phosphate buffer. The increase in fluorescence was monitored at the indicated times (Ex. 485 nm and Em. 520 nm). All values represent the mean ± SD of three individual experiments (p < 0.05, one-way ANOVA).