Figure 9. Model for origin of pathway variability.
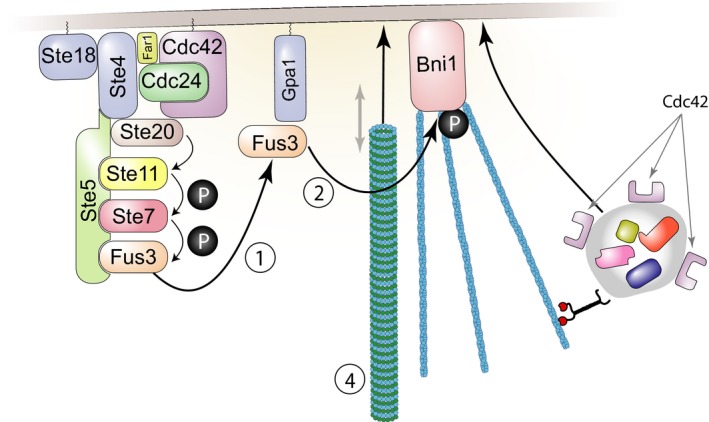
Our work suggests that disruption of microtubule plus‐end function near the signaling site by perturbations such as the Δbim1 mutation causes variability in Ste5 recruitment or Fus3 signaling. Figure shows stimulatory reactions at the signaling site by which irregularities in Ste5 recruitment or Fus3 signaling could be amplified. (1) Fus3 is phosphorylated and activated due to the operation of the PRS. Some phosphorylated Fus3 binds to the Gpa1 subunit of the dissociated G‐protein. (2) Gpa1‐bound Fus3 activates a protein, Bni1, which nucleates formation of actin cables. (3) Additional proteins involved in signaling, cell polarization, and cell fusion, including Cdc42 and Fus2 (Paterson et al, 2008, not shown) are then trafficked to the membrane as cargo carried along the actin cables. Activated Cdc42 stimulates its own activation (Kozubowski et al, 2008; Johnson et al, 2011). (4) Microtubule plus ends are captured by Kar3/Cik1 associated with Gpa1 (not shown) and Kar9/Bim1‐complexed plus ends can be walked down the actin cables to the signaling site by motor proteins including Myo2 (not shown). Microtubules, actin cables, and cargo proteins are larger than the approximate scale used here would suggest. Appendix and Appendix Fig S7 describe additional stimulatory reactions and positive feedbacks that might contribute to irregular signaling at the site.
