Fig. 3. Proposed AAC-VIa mechanism.
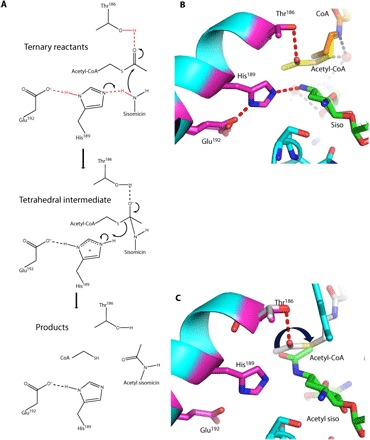
(A) Proposed catalytic mechanism of AAC-VIa–mediated acetylation of sisomicin. (B) Close-up view of the active site of the ternary enzyme-CoASH-sisomicin complex superimposed on the binary enzyme–acetyl-CoA complex. Active-site residues are colored with magenta carbon atoms, CoASH is colored with orange carbon atoms, acetyl-CoA is colored with yellow carbon atoms, and sisomicin is colored with green carbon atoms. (C) Close-up view of the active site of the enzyme–acetyl sisomicin complex superimposed on the binary enzyme–acetyl-CoA complex. Active-site residues are colored with magenta carbon atoms, acetyl-CoA is colored with gray carbon atoms, and acetylated sisomicin is colored with green carbon atoms. Hydrogen bonds are represented as red dashed lines.
