Fig. 1. Schematic representations of the experimental design and procedures.
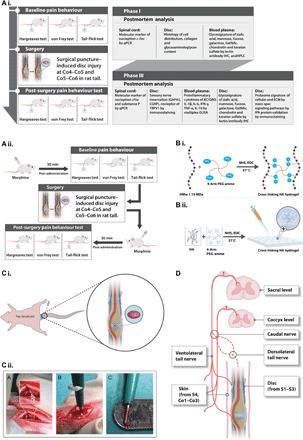
(A) Experimental design. (i and ii) The study was designed as follows: phase I, development and characterization of an IVD pain model; phase II, validation of the model using morphine; phase III, therapeutic efficacy of implanted HA hydrogel following IVD injury. (B) Cross-linking of high–molecular weight HA and four-arm PEG amine. (i) After functionalization with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), the amine groups of HA molecules react with the succinimidyl groups of PEG amine. (ii) Hydrogels for implantation were prepared by pipetting 4 μl of a mixture of HA, PEG amine, NHS, and EDC onto a hydrophobic surface, followed by incubation at 37°C for 1 hour to complete cross-linking. (C) Surgical procedure. (i) Identification of the coccygeal disc at the Co4–Co5 level. A rubber band (marked in blue) was applied at the base of the tail. Discs were treated by injury alone or injury with implantation of HA hydrogel (marked in purple). (ii) Steps of the surgical procedure. A disc was dissected by pushing aside connective tissue and tendons until the ivory matter of the AF tissue (gray arrow) was reached. The defect was created by puncturing NP tissue through AF tissue at a diameter of 1 mm and a depth of up to 2 mm. (D) Neuroanatomy of the rat tail to define the receptive fields of the stimuli used for the pain behavior tests.
