Abstract
The prospects for cell replacement in spinal cord diseases are impeded by inefficient stem cell delivery. The deep location of the spinal cord and complex surgical access, as well as densely packed vital structures, question the feasibility of the widespread use of multiple spinal cord punctures to inject stem cells. Disorders characterized by disseminated pathology are particularly appealing for the distribution of cells globally throughout the spinal cord in a minimally invasive fashion. The intrathecal space, with access to a relatively large surface area along the spinal cord, is an attractive route for global stem cell delivery, and, indeed, is highly promising, but the success of this approach relies on the ability of cells (1) to survive in the cerebrospinal fluid (CSF), (2) to adhere to the spinal cord surface, and (3) to migrate, ultimately, into the parenchyma. Intrathecal infusion of cell suspension, however, has been insufficient and we postulate that embedding transplanted cells within hydrogel scaffolds will facilitate reaching these goals. In this review, we focus on practical considerations that render the intrathecal approach clinically viable, and then discuss the characteristics of various biomaterials that are suitable to serve as scaffolds. We also propose strategies to modulate the local microenvironment with nanoparticle carriers to improve the functionality of cellular grafts. Finally, we provide an overview of imaging modalities for in vivo monitoring and characterization of biomaterials and stem cells. This comprehensive review should serve as a guide for those planning preclinical and clinical studies on intrathecal stem cell transplantation.
Introduction
Central nervous system (CNS) diseases and injuries are some of the most devastating for patients. The complexity and role of the CNS is such that its functional deterioration results in a huge impact on the quality of life, as well as an enormous financial burden to society. Cellular degeneration and death are the most common features of CNS disorders. In that way, several approaches that have attempted to regenerate cells, tissues, or organs in order to restore or establish normal function have been studied. In many instances, transplanted stem cell suspensions were shown to be highly therapeutic in small-animal models,1 but that was attributable to the broad distribution of transplanted cells in the CNS.2 The attempt to translate these exciting results to the clinical scenario has been challenging. While several clinical trials report therapeutic benefit,3,4 many other trials report good safety profile but no efficacy,5–7 triggering the closing of some cell-manufacturing companies. Such disappointing clinical translation results can be attributed to the large difference in the size of the CNS between mice and humans, as the mouse brain is 1000 times smaller. The issue of cell distribution in the large CNS must be addressed prior to the pursuit of more clinical research. Herein, we discuss the current clinical needs and solutions that have been used in cell-based therapies, with a particular focus on targeting the spinal cord. Recent reports dealing with hydrogels and nanoparticles for cell delivery to the CNS are also reviewed. The modulation of the microenvironment of cell-laden hydrogels with the use of nanoparticles and engineering strategies to allow in vivo imaging are also discussed in depth.
Targeting the spinal cord: clinical needs and solutions
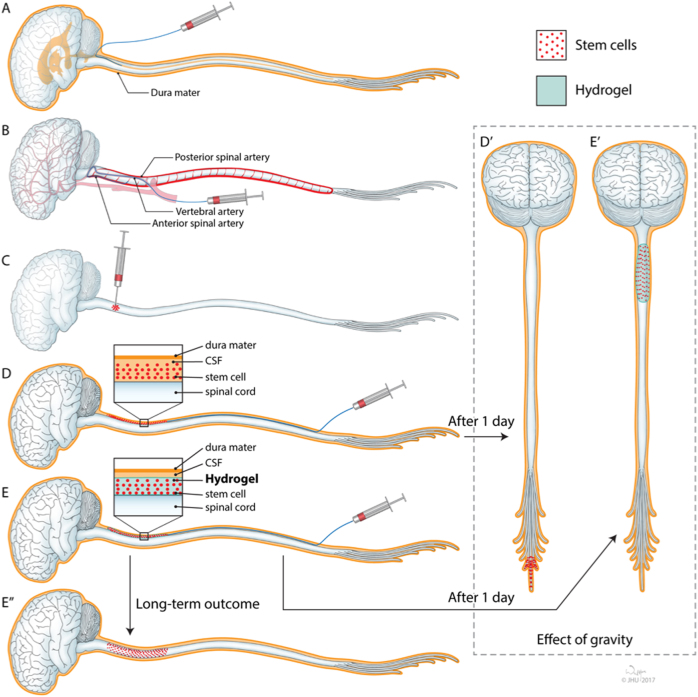
Intraventricular8 and intra-arterial9 routes are very promising for the delivery of stem cells to the brain. However, efficient delivery of stem cells to the broad areas of the spinal cord needs still to being resolved. There are several gateways to the spinal cord that have been considered, including the central canal, the intra-arterial, the intraparenchymal, and/or the intrathecal routes. Schematic representation of the cell/biomaterial constructs delivery routes into the spinal cord is depicted in Fig. 1.
Fig. 1.
Injection routes of stem cell/biomaterial constructs into the spinal cord
Central canal
The central canal of the spinal cord, an extension of the ventricular system, is a relatively narrow space, which also plays a central role in the CSF circulation. The obstruction of the cerebrospinal fluid (CSF) circulation following injection of stem cells could lead to a very debilitating disorder, syringomyelia,10 and thus, this route of cell delivery should be pursued clinically only after extensive research on large animals (Fig. 1a).
Intra-arterial
Blood for the spinal cord is supplied by a number of small segmental arteries, which are difficult to reach with an endovascular catheter, and, importantly, the obstruction of these arteries can result in serious and disabling consequences.11 Considering that most of the potential targets for therapy are within the cervical spine, any vascular occlusion or injury in this area may result in severe neurological deficits that could affect most of the body, including tetraplegia. In this context, the intra-arterial route for cell delivery to the spinal cord should be considered with extreme caution (Fig. 1b).
Intraparenchymal
Direct needle injections, including multi-site injections, are currently the most actively pursued strategy with which to deliver stem cells to the spinal cord, as it has been shown to be effective in small animals.12 While the procedure has been shown to be safe in large animals13,14 and open-label phase I/II clinical trials,15,16 the puncture of the spinal cord is a very complex and expensive procedure. Intraparenchymal delivery is also not well suited for disorders with global or multifocal pathology, as each injection delivers cells to only a relatively small volume of tissue. Multiple injections might be considered to improve cell biodistribution; however, that amplifies the risk of damage and any potential therapeutic effects could be offset by the procedure-related morbidity. The need for neurosurgical virtuosity and specialized injectors might limit the widespread application of this route. The invasive nature of intraparenchymal injection requires the ability to gain surgical access to the spinal cord, including muscle dissections and multilevel laminectomies. In addition, repeated needle insertion increases the risk for injury of a parenchymal vessel, with the resultant neurological consequences. Therefore, this approach is another example where direct translation of therapeutic effectiveness from small animals to patients may be challenging (Fig. 1c).
The spinal cord is thus a difficult target in which to achieve effective, global delivery of stem cells and there is a clear clinical need for a robust, minimally invasive, and safe method for efficient and repeatable stem cell delivery to the spinal cord. The low volume-to-surface ratio (in contrast to the brain) and easy access speaks in favor of using the extraspinal space as a route of cell delivery.
Intrathecal
The extraspinal space is filled with a CSF, and, most importantly, can be easily approached through a lumbar puncture, a method routinely used to sample the CSF, for drug delivery, as well as to lower CSF pressure during neurosurgical procedures. The use of widely available fluoroscopic guidance allows placement of a catheter tip at the desired position, such as the cervical area.17 Thus, if this route were effective, it could immediately be applied worldwide; however, several challenges need to be addressed before this approach can become a clinical reality. The most important is that the cells deployed to the CSF need to traverse the pia matter and enter the parenchyma of the spinal cord in the desired region. There is strong evidence about ability of the cells to migrate from the CSF compartments into parenchyma on early stage of development;2 however, adult or aging pia matter will likely be more challenging barrier to traverse. Some potential solutions to this challenge could be engineering the cells for expression of metalloproteinases18 or supplementation of biomaterials with enzymes selectively targeting collagen and reticular fibers of pia. The second challenge is that the cells injected as a suspension (Fig. 1d) settle due to gravity, and, in humans, usually accumulate below the spinal cord around the cauda equina (Fig. 1d’). Potentially, both challenges, i.e., adhesion to the spinal surface and sedimentation can be addressed by embedding cells within the hydrogel-based scaffold (Fig. 1e,e’,e”). This, in turn, calls for providing an appropriate environment that would match the requirements of the cell type and could be addressed by the use of nanoparticles that could slowly release the desired trophic factors. In subsequent sections, we will extensively review the options for the biomaterials (hydrogels and nanoparticles) to address the unmet need for stem cell delivery to the spinal cord via the intrathecal space.
Hydrogels as scaffolds for stem cell delivery
The application of appropriate scaffolding biomaterial matrices has gained a new impetus to repair the CNS because of the huge developments in cell engineering and cell-based treatment solutions. Scaffolds, by including natural extracellular matrix (ECM) proteins, can direct cell behavior by providing cues about cells during migration, differentiation, and regeneration in the CNS environment.19 In particular, the use of hydrogel systems is an attractive approach for stem cell delivery, as these systems can serve as temporary mimetic niche with which to support the survival of transplanted cells or recruited endogenous cells at the lesion site to promote recovery.20 The high-water content and tissue-like mechanical properties of hydrogels make them highly attractive scaffolds for implantation in soft tissue.21 Furthermore, the hydrogels present porous structures that allow cell attachment and growth, as well as the “smart” release of biological agents at the injury site.21,22
With respect to application in the spinal cord, hydrogels are the system of choice to provide the appropriate mechanics for precise localization and spatio-temporal control of cell delivery, and that is particularly important in case of transplantation to the intrathecal space. The rationale for using injectable hydrogels for intrathecal stem cell delivery is based on the observations that stem cells transplanted into the CSF are subject to gravitational sedimentation.8 In addition, cells injected into the CSF may lack cell–cell contact and RGD signaling. Indeed, it has been shown that RGD signaling provided as part of the biomaterial system improves cell survival and function.23 The quality of hydrogels that is critical, with respect to their use in highly sensitive tissues of the CNS, is that they are biocompatible, minimizing adverse tissue reaction in vivo.24 Hydrogels can be easily tuned (composition and functionalization) in order to facilitate injectability. Their biomechanical properties can be adjusted for smooth flow with low resistance during infusion, as well as to broadly mimic the host microenvironment composition, including physical and mechanical characteristics representative of native CNS tissue, which minimizes mechanical mismatch.25
Both physical and covalently cross-linked hydrogels have been applied to the treatment of spinal cord injury (SCI). Until now, only a few studies reported the development of injectable hydrogel systems for the intrathecal delivery of cells to the CSF. Physical gels are simpler to use, since they typically do not involve harsh cross-linking conditions, thus reducing the possible toxicity normally associated with the use of covalent coupling agents.26 In fact, these types of gels often require a gelation-triggering system, such as local cooling,22 which introduces the risk of tissue damage, and, in case of intrathecal injection, is not applicable.
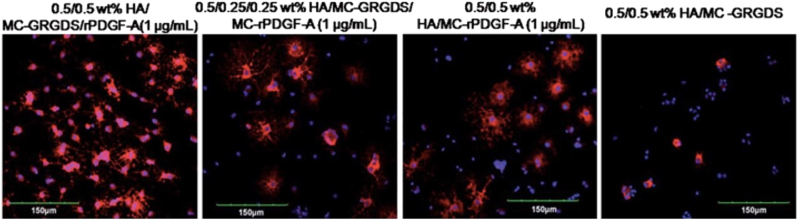
Chemically cross-linked hydrogels, however, are relevant because they present more desirable properties than physical gels in terms of flexibility to manipulate physical and biological properties. Synthetic functional groups can be incorporated in the polymers to enable (or enhance) gelation, for improved in vivo stability, or to enhance specific enzymatic susceptibility of the hydrogels.27 Chemically cross-linked hyaluronan is an example where researchers exploited the biocompatible, biodegradable, angiogenic, and anti-inflammatory nature of the base polymer. For example, Gupta et al.28 described, for the first time, the physical blend of an HA and methyl cellulose (HAMC)-injectable hydrogel to deliver NSCs into the rat brain after a clip compression injury. Also, Mothe et al.29 examined the survival and efficacy of adult brain-derived neural stem/progenitor cells (NSPCs) injected within a modified HAMC hydrogel with recombinant rat platelet-derived growth factor-A (rPDGF-A). The authors showed that, while only a limited number of surviving cells was found, the hydrogel promoted the survival of host neurons and oligodendrocytes, which was associated with better functional recovery on the ladder walk.29 Further, the same authors reported that the modification of the previously used HAMC with RGD promoted the survival, integration, and differentiation of human pluripotent stem cell-derived oligodendrocyte progenitor cells30 (Fig. 2). While over-proliferation of the cells was reported in that study, it demonstrated that the modified HAMC hydrogel reduces tumor formation by promoting differentiation in vivo. Control animals that received cells without hydrogel demonstrated extensive tumor formation and a decline in motor function.31 A combination of peptide-modified gellan gum (GG-GRGDS) hydrogels also showed a great potential to be used in cellular therapies designed to treat SCI. A co-culture of adipose stem cells (ASCs) and olfactory ensheathing cells (OECs) encapsulated into the GG-GRGDS hydrogel resulted in significant motor and histological improvements of SCI in rats.32
Fig. 2. Effect of immobilization of GRGDS peptide on HAMC hydrogels for the differentiation of rat NSPCs.
Confocal images of rat NSPCs after encapsulation in 0.5/0.5 wt% HAMC gels for 7 days. Cells were stained for anti-RIP (for oligodendrocytes, red) and counterstained with DAPI (for cell nuclei, blue). Reprinted from Tam et al.30
In brief, the selection of biomaterials, the surface morphology of the substrates, neurotrophic factors, cell density, and the effect of serum and many other factors can all affect the proliferation and differentiation of cultured neural stem cells. When envisioning the design of the cell culture environment in vitro and in vivo, as many factors as possible related to biomaterials should be considered and optimized to achieve the best biological performance, in the short-term, medium-term, and long-term. All these studies provide a promising strategy, combining a biocompatible and injectable polymer and stem cells to form an effective cell transplantation system for the treatment of spinal cord diseases. In order to develop more efficient treatment solutions, future research should focus on optimizing the conditions for augmenting stem cell survival, proliferation, migration, differentiation, and integration into the host tissue.
Nanoparticles for the modulation of the microenvironment of cell-laden hydrogels
One of the most important characteristics of hydrogels that determine local microenvironment and injectability are their rheological properties33 and it has been shown that the rheological properties of a given hydrogel vary.34 When a hydrogel is injected, its rheological behavior and viscoelastic properties (storage modulus and loss modulus, G’ and G”, respectively) are negatively affected. In order to improve the viscoelastic properties, without altering the gel-like behavior of both acellular and cell-laden hydrogels, nanoparticles have been used as a reinforcement or filler material.33,35
In fact, the use of nanoparticle-based systems has expanded interest because of the exciting prospects.36 The use of materials at the nanoscale provides the extraordinary possibility to modify some of the properties of therapeutic carriers, such as solubility, diffusivity, biodistribution, release characteristics, and immunogenicity. In addition, these kinds of carriers show a longer circulation half-life, superior bioavailability, and lower toxicity.37,38
Despite the large number of nanocarriers that are being developed and investigated for specific drug delivery purposes in the CNS, only a few studies have reported its application in the CSF. The vast majority of NPs for the CNS range from classical linear polymers to novel spherical molecules. For example, dendrimers that have a spherical morphology and highly branched have been shown to be interesting nanocarriers for CNS applications. They facilitate surface functionalization and control over their size, which influences the drug payload and targeting features.39,40 There are different dendrimers that have been exploited extensively for drug delivery, including poly(amidoamine) (PAMAM), poly(etherhydroxylamine) (PEHAM), and poly(propyleneimine) (PPI) dendrimers.41 Because of their unique structures and properties, PAMAM dendrimers have been the most investigated for the use in CNS applications. In fact, Oliveira et al. reported a new functionalization for a PAMAM dendrimer, by grafting it to a carboxymethylchitosan (CMCht) to improve the loading capacity.42 That nanocarriers were shown to be internalized by primary neurons and glial cells in culture system, suggesting their utility for CNS indications39 (Fig. 3). In another study, the corticosteroid, methylprednisolone (MP), was incorporated into the CMCht/PAMAM and administered directly into the CSF in the cisterna magna of Wistar rats. This study showed that MP-loaded CMCht/PAMAM broadly diffused in the healthy rat brain following administration in the CSF, while delivering MP. The incorporation of MP into a dendrimer formulation was responsible for modulation of the metabolic activity of microglia.43 Recently, a few studies have reported on another approach using magnetic nanoparticles (MNPs) for magnetic guidance of drugs and their targeted delivery.44,45 Lueshen et al.46 developed a physiologically and anatomically consistent in vitro human spine model that reproduced natural CSF pulsations to infused gold-coated magnetite nanoparticles. This system allowed a more targeted drug delivery to specific regions using an external magnetic field. The same authors proved the increase in targeting efficacy using magnetizable implants.47 More recently, they validated this intrathecal magnetic drug targeting (IT-MDT) approach in vivo using Sprague-Dawley rats.48
Fig. 3. Internalization experiments within cortical glial cell cultures.
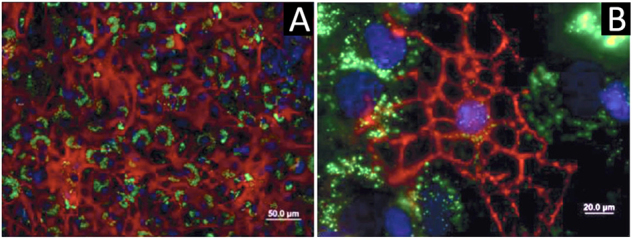
a Astrocytes were able to internalize the FITC-labeled CMCht/PAMAM dendrimer nanoparticles after 48 h of incubation. b Oligodendrocytes also were able to internalize the FITC-labeled CMCht/PAMAM dendrimer nanoparticles. Representative image of the nanoparticles distributed along the intracellular compartment. Adapted from Salgado et al.39
With regard to the linear polymers, one of the most widely used for drug delivery is poly(d,l-lactic-co-glycolic acid) (PLGA), since it is biodegradable and there is minimal systemic toxicity associated with CNS applications.49 Minocycline-loaded PLGA nanoparticles showed no in vitro degradation of the drug in artificial CSF during the seven-day stability study.50
In addition to the use of nanoparticles for drug or growth factor delivery systems, nanoparticles have gained special attention for stem cell tracking, namely, MNPs for detection by MRI. Cell tracking is important, as it allows for a better understanding and optimization of cell-mediated effects.51 Meta-analyses of cell-mediated effects in preclinical cell therapy studies in neurological disorders have revealed the primary therapeutic mechanisms52 and nanotechnology can offer a non-invasive monitoring system for further improving these positive effects.
Non-invasive imaging of intrathecally injected biomaterials
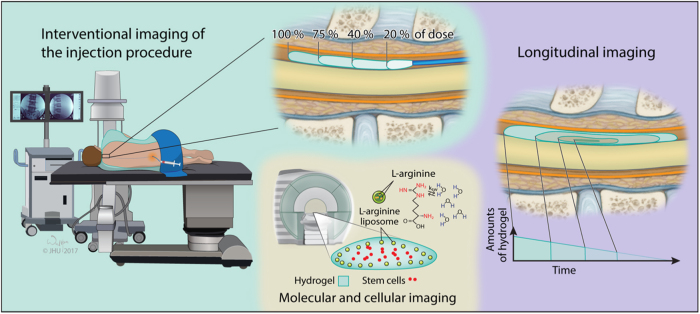
The advantages of injecting cells via intrathecal route are clear as discussed above. The challenge, however, is related to the uncertainty about cell biodistribution and potentially high variability of biomaterial/cell dispersal. While direct intraparenchymal injection results in highly reproducible and rather predictable placement,53 in case of injection into the fluid compartments uncertainty is high. This uncertainty is related to the mixing and dilution of biomaterial with CSF and its redistribution with fluid circulation. In this context, it is highly desirable to monitor injected biomaterials using non-invasive imaging. Even though literature on image-guided intrathecal injection of biomaterial/cell composites is very limited, application of this approach in other organ systems is quite abundant thus here we will focus on the rationale and the needs of imaging for intrathecal injection, and, will suggest imaging strategies that are best suited to fulfill these needs. There are three areas where imaging could assist in improving the success of intrathecal biomaterial/cell injection including monitoring the procedure of biomaterial injection in real-time, longitudinal assessment and finally, molecular imaging of the local microenvironment at the injection site (Fig. 4).
Fig. 4.
Non-invasive imaging of intrathecally injected cell/biomaterial constructs
Imaging is expected to provide a breadth of information about the biomaterial itself, the embedded cells, and the graft-tissue interactions in the context of the healing process. Moreover, it is important that imaging requirements, such as incorporation of the contrast agent, does not alter the properties of the biomaterial, does not interfere with biology of embedded cells, and, ideally, does not compromise functional assessment by any imaging modalities.54 The selection of the optimal imaging technique for a particular application is not a trivial task, and multiple factors have to be considered. One example is the case of a superficial injection within a few millimeters under the skin, in which a low-energy imaging method (e.g., photoacoustic, fluorescent or bioluminescent) is sufficient to track hydrogel integrity and release of therapeutics within the injected organism.55,56 However, to monitor delivery into deep structures, such as intravascular or intra-organ injection, including the intrathecal space, tomographic techniques (e.g., MRI, PET, and X-ray) are usually required.57–59 Each imaging modality has unique properties with advantages and disadvantages; here, we will review these features as they relate to placement of biomaterials in the intrathecal space.
Interventional imaging of the injection procedure
Placement of the intrathecal catheter at the desired level of the spinal cord is performed under fluoroscopic visualization and this procedure is routinely performed clinically with an example of baclofen pump implantation for treatment of dystonia.60 Infusion of drugs as fluid suspension can be performed without complications even chronically over weeks or months, as they simply mix with the CSF. Implantation of hydrogels, however, is more demanding and requires more careful approach as it is desired that the material would persist locally in a specific and selected area of the intrathecal space over the required period of time. When biomaterial is being injected, dynamic imaging can be used to assure the precision of targeting and to minimize the risk of excessive injection or misplacement. Indeed, misplacement of the biomaterial may have negative consequences beyond the suboptimal therapeutic effect, as misinjected graft could lead to unwanted adverse effects. Infusion into intrathecal space results in its rostral or caudal spread from the catheter tip, or combination of the two. Without imaging in real-time, proper placement is practically impossible. Monitoring biomaterial biodistribution can be accomplished with the use of X-ray. X-ray imaging based on phase contrast proved to be capable of detecting the hydrogel structure without the addition of contrast agent. The nonporous and porous PEG hydrogels were discernible from surrounding water or soft tissue in vitro without the use of contrast agents.61 In addition, Faraj et al.62 demonstrated that high-resolution computed tomography (micro-CT) enables the determination of the structure of soft scaffolds in vitro. In addition to low X-ray attenuation of collagen-based hydrogels, different combinations of contrast agents were used. It was shown that the application of osmium tetroxide and uranyl acetate, or a combination of uranyl acetate and lead citrate, allowed high-resolution 3D imaging of the structure of the scaffolds. Lei et al.63 showed the possibility of non-invasive tracking of radiopaque thermo-reversible hydrogels after implantation, and the opportunity to obtain detailed 3D morphological information in a real-time manner. Due to its accessibility and low cost, X-ray imaging techniques are promising tools for hydrogel observation but ionizing radiation is a concern and the motivation for developing other imaging modalities such as MRI. Indeed MRI is extensively used in biomedicine2,64 and recently significant progress has been made in interventional MRI including real-time assessment of injected cells65 and biomaterials.66 MRI has many features that make it one of the most desirable and preferable imaging modalities for imaging in biomedicine. These features include excellent soft tissue contrast, tomographic capabilities, excellent anatomical information, and it is non-invasive and radiation-free. MRI has been widely used for in vivo and ex vivo analysis of biomaterials and transplanted cells, and, while detection of native components of hydrogel has been demonstrated,67 labeling with MRI contrast agent is usually required. Imaging moiety is often introduced in the form of a nanoparticle such as iron oxide68 gadolinium69,70 or fluorine nanoemulsions.71 Various types of nanocarriers have been developed including more advanced systems with both therapeutic and diagnostic components (Theranostic agents). Good example of such theranostic agents in the contex of regenerative medicine are iron oxide-based particles loaded with microRNAs for simultaneous tracking and manipulation of transplanted cells.72 Another application of nanoparticles for imaging is by combining contrast mechanism for more than one modality known as multimodality nanoparticles, such as a combination of PET, near-infrared fluorescence, and MRI73 While there are no published reports on intrathecal injection of biomaterials guided by dynamic imaging we would like to review several relevant applications based on imaging of labeled stem cells.
Superparamagnetic iron oxide nanoparticles (SPION) are of particular interest for stem cell tracking, since they, in general, do not result in adverse effects on cell survival and functionality in terms of differentiation capacity, gene expression profiles, or migratory capacity.8,74 Bigini et al.75 labeled human fetal cells with SPIO nanoparticles in the brain of mice with motor neuron disease. It was reported that transplanted cells rapidly diffused throughout the fourth ventricle at the level of the spinal cord, and the labeling did not affect the proliferation and metabolic activity of cells.75 MRI has been successfully used for the dynamic assessment of cell biodistribution in real-time during intra-arterial injection in rodents76 where imaging was instrumental in optimizing the procedure of cell delivery. Recently, real-time MRI monitoring has been applied to predict and improve the precision of cell delivery into the brain in small-animal and large-animal models.2 While real-time assessment of intrathecal injection of the biomaterial with MRI has not been reported it is highly feasible and application of this technique would likely provide important clues on how to assure reproducibility, reliability and efficacy of intrathecal biomaterial/cell delivery.
Longitudinal imaging
While most of the requirements for imaging of biomaterials over time are common with described above real-time imaging, there are few unique challenges related to specificity. The advantage of interventional imaging is an access to pre-injection baseline and unequivocal interpretation of post injection images following simple subtraction process. Longitudinal imaging introduces uncertainty as some pathological processes may imitate signal features of the graft with an example of hypointense signal produced by both iron oxide nanoparticle-based contrast agents and a hemorrhage.77 Nevertheless both X-ray and MRI has been used for monitoring injected biomaterials over time. One example is a study by Appel et al.78 showing that X-ray phase contrast (XPC) CT application allows 3D visualization and quantification of hydrated soft tissues and PEG hydrogels in vivo, with no contrast agents. The XPC CT imaging enabled a clear distinction between surrounding tissue after transplantation and the hydrogel structure. Also, tantalum, known for its biological properties of facilitating soft tissue regeneration and vascularization, is, at the same time a promising contrast agent for X-ray imaging, enhancing the visibility of cells and biomaterial grafts.79 MRI has been shown instrumental in the assessment of biomaterials biodegradability in a study reported by Yang et al..80 That work showed that the biodegradability level of hydrogels can be monitored and quantified in vivo using an alteration of 19F intensity. Recently, MR imaging of hydrogels transplanted as scaffolds, as well as an injectable formulation, has been reported.81 Zhang et al.82 presented in situ, cross-linkable, HA-based hydrogels, hybridized with iron oxide nanoparticles to enable their detection on MRI. Another interesting study used iron oxide as a contrast agent and multiple crystals of iron oxide were encapsulated inside the polyacrylamide matrix, yielding very high relaxivity. Because of the monitoring of the amount of liberated iron oxide nanoparticles by the hyaluronidases, it was possible to monitor and analyze the hydrogel degradation.83 Bible and co-workers84 showed non-invasive imaging of the ECM scaffold in a stroke-damaged rat brain using 19F MRI, demonstrating that transplantation of neural stem cells embedded in xenogeneic ECM scaffolds resulted in uniformly distributed cells throughout the lesion cavity.
Molecular and cellular imaging of the local microenvironment
One of the most challenging but highly rewarding tasks is non-invasive imaging of the local microenvironment reporting on the molecular processes within and around the injected biomaterial. Greatest potential in that area has recently developed molecular MRI technique that is based on chemical exchange saturation transfer (CEST-MRI), which is a novel molecular MRI technique with the important advantage that components which naturally occur in the biomaterials are used to generate contrast.85 Endogenous molecules dependent on the content of labile protons in the sample may generate CEST contrast, thus facilitating their detection by CEST-MRI without any additional contrast agent. Liang et al.67 reported on the use of CEST-MRI to monitor biodegradation of a gelatin-containing HA-based hydrogels, both in vitro as well as in vivo in mice. In addition, Jin et al.86 described the use of CEST for both in vitro and in vivo examination of ECM hydrogels in a rat stroke model. In vitro CEST imaging was instrumental in demonstrating dynamic changes in the different components of the ECM inside the hydrogel. Moreover, in vivo CEST examination allowed detection of ECM hydrogel distribution and degradation that strongly corresponded to histological studies post mortem.86 Chan et al.57 described pH nanosensor-based magnetic resonance imaging as a modality with which to image encapsulated cell death in vivo. Ultrapure, low-viscosity, high-guluronate alginate and ultrapure, low-viscosity, high-mannuronate alginate (NovaMatrix), and liposomes containing l-arginine, were used for microcapsule preparation (Fig. 4). It was shown that LipoCEST nanosensors were sensitive enough to detect cell death caused by incomplete immunoprotection.
In the context of monitoring local cellular microenvironment it is important to mention bioluminescence imaging (BLI). This technique is based on a reporter gene (e.g., firefly luciferase) that is expressed by the cells of interest. As the photon signal is generated only by metabolically active cells and is dependent on access to ATP, it is an excellent method for in vivo monitoring of cell viability. Even though this modality is only applicable to small-animal models it is invaluable for assessment of biomaterial/cell composites. As described by Allen et al.87 GFP/Luc hMSCs were embedded in (low-molecular-weight-irradiated RGD-functionalized) alginate or in (SeaPlaque) agarose hydrogels and injected subcutaneously in rats. Although both hydrogel types showed a linear correlation between the BLI signal and the live cell number using a 30-min imaging protocol, there was a difference in the magnitude of the BLI signal measured between the agarose and alginate materials, exemplifying the utility of BLI for monitoring interactions between biomaterials and embedded cells. In addition, Liang et al. showed that BLI is useful for monitoring the viability of cells embedded in injectable hydrogel.88 That study showed that HA-based hydrogels improved the survival and proliferation of three different transplanted cell lines (C17.2 neural stem cells, ReNcells, and glial progenitors). Similarly, Levit et al. used Luc hMSCs encapsulated in a low-viscosity guluronic acid-based alginate (LVG, Novamatrix) in a rat myocardial infarction model.89 BLI showed that encapsulation of MSCs in an LVG hydrogel minimized scar formation and improved cardiac function. Similarly, luciferase-expressing ASCs (Luc ASCs) embedded within fibrin scaffolds and transplanted in the injured heart were tracked with BLI as reported by Yang et al.90
Overall, several imaging modalities offer unique opportunities for non-invasive monitoring of the intrathecal injection. Each of the modalities has strengths and limitations with multimodality approach likely required for providing most comprehensive information.
Concluding remarks and future trends
The spinal cord is clearly one of the most difficult targets for the transplantation of stem cells and one of the most challenging elements is the route of cell delivery. Diseases characterized by disseminated pathology require global cell engraftment, and, in these cases, intrathecal injection seems to be highly recommended. The infusion of cell suspensions directly into the CSF can result in cell sedimentation and suboptimal cell biodistribution, and hence, the need for supporting biomaterials. The transplantation of cells embedded in injectable hydrogels addresses the problem of possible cell sedimentation while mimicking the native extracellular matrix, additional benefits of hydrogels include bioadhesiveness and offer the potential for supplementation with nanocarriers laden with molecules that can promote survival and differentiation of stem cells. Finally, imaging technology offers unique opportunities for the characterization of cell-biomaterial composites both in vitro and after transplantation.
As shown above, there have been several reports that advocate the use of the intrathecal route for stem cell delivery, and progress in the field of biomaterials is unprecedented, offering a breadth of injectable hydrogel materials that meet the requirements of intrathecal injection. While the applications for hydrogel-embedded stem cells for intrathecal transplantation are limited, we certainly hope this review will spark more interest in this exciting area. Recent progress in the non-invasive imaging of biomaterials, and, particularly, clinically applicable modalities, such as MRI, will help guide the development of more effective and safer protocols for intrathecal therapeutic cell transplantation.
Acknowledgements
We are grateful for the funds provided under the project NanoTech4ALS (ref. ENMed/0008/2015, 13/EuroNanoMed/2016), funded under the EU FP7 M-ERA.NET program, Strategmed 1/233209/12/NCBIR/2015, and NIH R01 NS091100. The FCT distinction attributed to J.M.O. under the Investigator FCT program (IF/01285/2015) is also gratefully acknowledged.
Author contributions
Conception and design of the manuscript: J.M.O., M.J., P.W. Drafting the manuscript: L.C., J.S.C., S.V., M.M., B.L., L.S., P.S., I.M.C., D.G., L.K., R.L.R. Critical revision of the manuscript: J.M.O., L.C., J.S.C., S.V., M.M., B.L., L.S., P.S., I.M.C., D.G., L.K., R.L.R., M.J., P.W. Final approval: J.M.O., L.C., J.S.C., S.V., M.M., B.L., L.S., P.S., I.M.C., D.G., L.K., R.L.R., M.J., P.W.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Windrem MS, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyczek A, et al. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp. Neurol. 2017;291:74–86. doi: 10.1016/j.expneurol.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kefalopoulou Z, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg, G. K., Kondziolka, D., Bates, D. & SB623 Stroke Phase 1/2A Study Group. Response by Steinberg et al. to letter regarding article, “Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2A Study”. Stroke 47, e269 (2016). [DOI] [PMC free article] [PubMed]
- 5.Hess DC, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KD, et al. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N, et al. Neural stem cell engraftment and myelination in the human brain. Sci. Transl. Med. 2012;4:155ra137–155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janowski M, et al. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS ONE. 2014;9:e97631. doi: 10.1371/journal.pone.0097631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walczak P, et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow. Metab. 2016;37:2346–2358. doi: 10.1177/0271678X16665853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunert P, Janowski M, Zakrzewska A, Marchel A. Syringoperitoneal shunt in the treatment of syringomyelia. Neurol. Neurochir. Pol. 2009;43:258–262. [PubMed] [Google Scholar]
- 11.Zhao Z, et al. The effect from different numbers of segmental arteries ligation to the spinal cord in the clinical practice of posterior vertebral column resection correction. Eur. Spine J. 2017;26:1937–1944. doi: 10.1007/s00586-017-5067-x. [DOI] [PubMed] [Google Scholar]
- 12.Lepore AC, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raore B, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine. 2011;36:E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez J, et al. Preclinical validation of multilevel intraparenchymal stem cell therapy in the porcine spinal cord. Neurosurgery. 2015;77:604–612. doi: 10.1227/NEU.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 15.Glass JD, et al. Transplantation of spinal cord-derived neural stem cells for ALS: analysis of phase 1 and 2 trials. Neurology. 2016;87:392–400. doi: 10.1212/WNL.0000000000002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi, A. D. et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery 10.1093/neuros/nyx250 (2017). [DOI] [PubMed]
- 17.Wahlig JB, Welch WC, Kang JD, Jungreis CA. Cervical intrathecal catheter placement for cerebrospinal fluid drainage: technical case report. Neurosurgery. 1999;44:419–421. doi: 10.1097/00006123-199902000-00118. [DOI] [PubMed] [Google Scholar]
- 18.Kanemitsu M, et al. EMMPRIN overexpression in SVZ neural progenitor cells increases their migration towards ischemic cortex. Exp. Neurol. 2017;297:14–24. doi: 10.1016/j.expneurol.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Assuncao-Silva RC, Gomes ED, Sousa N, Silva NA, Salgado AJ. Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells Int. 2015;2015:948040. doi: 10.1155/2015/948040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim JB, Ankeny RF, Kim H, Nerem RM, Khang G. A study of a three-dimensional PLGA sponge containing natural polymers co-cultured with endothelial and mesenchymal stem cells as a tissue engineering scaffold. Biomed. Mater. 2014;9:045015. doi: 10.1088/1748-6041/9/4/045015. [DOI] [PubMed] [Google Scholar]
- 21.Tan J, Gemeinhart RA, Ma M, Saltzman WM. Improved cell adhesion and proliferation on synthetic phosphonic acid-containing hydrogels. Biomaterials. 2005;26:3663–3671. doi: 10.1016/j.biomaterials.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 22.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Adil MM, et al. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials. 2017;136:1–11. doi: 10.1016/j.biomaterials.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Ballios BG, et al. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell. Rep. 2015;4:1031–1045. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed. Mater. 2012;7:024101. doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- 26.Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015;21:298–313. doi: 10.1089/ten.teb.2014.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34:3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Tam RY, Cooke MJ, Shoichet MS. A covalently modified hydrogel blend of hyaluronan-methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J. Mater. Chem. 2012;22:19402–19411. doi: 10.1039/c2jm33680d. [DOI] [Google Scholar]
- 31.Fuhrmann T, et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Gomes ED, et al. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials. 2016;105:38–51. doi: 10.1016/j.biomaterials.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Correia J, et al. Rheological and mechanical properties of acellular and cell-laden methacrylated gellan gum hydrogels. J. Biomed. Mater. Res. A. 2013;101:3438–3446. doi: 10.1002/jbm.a.34650. [DOI] [PubMed] [Google Scholar]
- 34.Kesselman D, Kossover O, Mironi-Harpaz I, Seliktar D. Time-dependent cellular morphogenesis and matrix stiffening in proteolytically responsive hydrogels. Acta Biomater. 2013;9:7630–7639. doi: 10.1016/j.actbio.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Russo T, et al. Systematic analysis of injectable materials and 3D rapid prototyped magnetic scaffolds: from CNS applications to soft and hard tissue repair/regeneration. Procedia Eng. 2013;59:233–239. doi: 10.1016/j.proeng.2013.05.116. [DOI] [Google Scholar]
- 36.Lu CT, et al. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014;9:2241–2257. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emerich DF, Thanos CG. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug. Target. 2007;15:163–183. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- 38.Wang AZ, et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert. Opin. Biol. Ther. 2008;8:1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira JM, Salgado AJ, Sousa N, Mano JF, Reis RL. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—a review. Prog. Polym. Sci. 2010;35:1163–1194. doi: 10.1016/j.progpolymsci.2010.04.006. [DOI] [Google Scholar]
- 40.Svenson S, Tomalia DA. Dendrimers in biomedical applications - reflections on the field. Adv. Drug Deliv. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira JM, et al. Surface engineered carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles for intracellular targeting. Adv. Funct. Mater. 2008;18:1840–1853. doi: 10.1002/adfm.200800165. [DOI] [Google Scholar]
- 43.Cerqueira SR, et al. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2016;12:972–972. doi: 10.1002/smll.201503492. [DOI] [PubMed] [Google Scholar]
- 44.Alexiou C, et al. Cancer therapy with drug loaded magnetic nanoparticles—magnetic drug targeting. J Magn. Magn. Mater. 2011;323:1404–1407. doi: 10.1016/j.jmmm.2010.11.059. [DOI] [Google Scholar]
- 45.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Lueshen E, et al. Intrathecal magnetic drug targeting using gold-coated magnetite nanoparticles in a human spine model. Nanomed. 2014;9:1155–1169. doi: 10.2217/nnm.13.69. [DOI] [PubMed] [Google Scholar]
- 47.Lueshen E, Venugopal I, Soni T, Alaraj A, Linninger A. Implant-assisted intrathecal magnetic drug targeting to aid in therapeutic nanoparticle localization for potential treatment of central nervous system disorders. J. Biomed. Nanotechnol. 2015;11:253–261. doi: 10.1166/jbn.2015.1907. [DOI] [PubMed] [Google Scholar]
- 48.Venugopal I, Habib N, Linninger A. Intrathecal magnetic drug targeting for localized delivery of therapeutics in the CNS. Nanomed. 2017;12:865–877. doi: 10.2217/nnm-2016-0418. [DOI] [PubMed] [Google Scholar]
- 49.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Holmkvist AD, Friberg A, Nilsson UJ, Schouenborg J. Hydrophobic ion pairing of a minocycline/Ca(2+)/AOT complex for preparation of drug-loaded PLGA nanoparticles with improved sustained release. Int. J. Pharm. 2016;499:351–357. doi: 10.1016/j.ijpharm.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Lucignani G, Rodriguez-Porcel M. In vivo imaging for stem cell therapy: new developments and future challenges. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:400–405. doi: 10.1007/s00259-010-1695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem. Cells. Dev. 2010;19:5–16. doi: 10.1089/scd.2009.0271. [DOI] [PubMed] [Google Scholar]
- 53.Walczak P, et al. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59:499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janowski M, Bulte JW, Walczak P. Personalized nanomedicine advancements for stem cell tracking. Adv. Drug. Deliv. Rev. 2012;64:1488–1507. doi: 10.1016/j.addr.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 2009;20:74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Short AR, et al. Imaging cell-matrix interactions in 3D collagen hydrogel culture systems. Macromol. Biosci. 2017;17:1600478. doi: 10.1002/mabi.201600478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan KW, et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat. Mater. 2013;12:268–275. doi: 10.1038/nmat3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan KW, et al. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. J. Control Release. 2014;180:51–59. doi: 10.1016/j.jconrel.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J, et al. Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angew. Chem. 2011;50:2317–2321. doi: 10.1002/anie.201007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson S, et al. Image-guided intrathecal baclofen pump catheter implantation: a technical note and case series. J. Neurosurg. Spine. 2017;26:621–627. doi: 10.3171/2016.8.SPINE16263. [DOI] [PubMed] [Google Scholar]
- 61.Brey EM, et al. X-ray imaging of poly(ethylene glycol) hydrogels without contrast agents. Tissue Eng. Part C Methods. 2010;16:1597–1600. doi: 10.1089/ten.tec.2010.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faraj KA, et al. Micro-computed tomographical imaging of soft biological materials using contrast techniques. Tissue Eng. Part C Methods. 2009;15:493–499. doi: 10.1089/ten.tec.2008.0436. [DOI] [PubMed] [Google Scholar]
- 63.Poort H, et al. Study protocol of the TIRED study: a randomised controlled trial comparing either graded exercise therapy for severe fatigue or cognitive behaviour therapy with usual care in patients with incurable cancer. BMC Cancer. 2017;17:81. doi: 10.1186/s12885-017-3076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin H, et al. Rabbit model of human gliomas: implications for intra-arterial drug delivery. PLoS ONE. 2017;12:e0169656. doi: 10.1371/journal.pone.0169656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barczewska M, et al. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS. One. 2013;8:e74658. doi: 10.1371/journal.pone.0074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnett BP, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat. Med. 2007;13:986–991. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 67.Liang Y, et al. Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials. 2015;42:144–150. doi: 10.1016/j.biomaterials.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley interdisciplinary reviews. Nanomed. Nanobiotechnol. 2011;3:343–355. doi: 10.1002/wnan.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicholls FJ, et al. DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. Biomaterials. 2016;77:291–306. doi: 10.1016/j.biomaterials.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nejadnik H, Castillo R, Daldrup-Link HE. Magnetic resonance imaging and tracking of stem cells. Methods Mol. Biol. 2013;1052:167–176. doi: 10.1007/7651_2013_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janjic JM, Ahrens ET. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leder A, et al. Micron-sized iron oxide-containing particles for microRNA-targeted manipulation and MRI-based tracking of transplanted cells. Biomaterials. 2015;51:129–137. doi: 10.1016/j.biomaterials.2015.01.065. [DOI] [Google Scholar]
- 73.Xie J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kedziorek DA, et al. Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn. Reson. Med. 2010;63:1031–1043. doi: 10.1002/mrm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bigini P, et al. Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease. PLoS ONE. 2012;7:e32326. doi: 10.1371/journal.pone.0032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrzejewska A, et al. Pre- and postmortem imaging of transplanted cells. Int. J. Nanomed. 2015;10:5543–5559. doi: 10.2147/IJN.S83557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rigol M, et al. Hemosiderin deposits confounds tracking of iron-oxide-labeled stem cells: an experimental study. Transplant. Proc. 2008;40:3619–3622. doi: 10.1016/j.transproceed.2008.05.084. [DOI] [PubMed] [Google Scholar]
- 78.Appel AA, et al. X-ray phase contrast allows three dimensional, quantitative imaging of hydrogel implants. Ann. Biomed. Eng. 2016;44:773–781. doi: 10.1007/s10439-015-1482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohandas G, Oskolkov N, McMahon MT, Walczak P, Janowski M. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol. Exp. 2014;74:188–196. doi: 10.55782/ane-2014-1984. [DOI] [PubMed] [Google Scholar]
- 80.Yang X, et al. Injectable hyaluronic acid hydrogel for 19F magnetic resonance imaging. Carbohydr. Polym. 2014;110:95–99. doi: 10.1016/j.carbpol.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 81.Kim JI, et al. Long-term theranostic hydrogel system for solid tumors. Biomaterials. 2012;33:2251–2259. doi: 10.1016/j.biomaterials.2011.11.083. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, et al. Injectable in situ forming hybrid iron oxide-hyaluronic acid hydrogel for magnetic resonance imaging and drug delivery. Macromol. Biosci. 2014;14:1249–1259. doi: 10.1002/mabi.201400117. [DOI] [PubMed] [Google Scholar]
- 83.Moffat BA, et al. A novel polyacrylamide magnetic nanoparticle contrast agent for molecular imaging using MRI. Mol. Imaging. 2003;2:324–332. doi: 10.1162/153535003322750664. [DOI] [PubMed] [Google Scholar]
- 84.Bible E, et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials. 2012;33:2858–2871. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J. Magn. Reson. 2013;229:155–172. doi: 10.1016/j.jmr.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin T, et al. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials. 2017;113:176–190. doi: 10.1016/j.biomaterials.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allen AB, Gazit Z, Su S, Stevens HY, Guldberg RE. In vivo bioluminescent tracking of mesenchymal stem cells within large hydrogel constructs. Tissue Eng. Part C Methods. 2014;20:806–816. doi: 10.1089/ten.tec.2013.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang Y, Walczak P, Bulte JW. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials. 2013;34:5521–5529. doi: 10.1016/j.biomaterials.2013.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levit RD, et al. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc. 2013;2:e000367. doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang JJ, et al. Real-time tracking of adipose tissue-derived stem cells with injectable scaffolds in the infarcted heart. Heart Vessel. 2013;28:385–396. doi: 10.1007/s00380-012-0275-0. [DOI] [PubMed] [Google Scholar]