Abstract
This study is to analyze the radiation dose of head, body and tail of hippocampus (HC) of nasopharyngeal carcinoma (NPC) patients treated with intensity modulated radiotherapy (IMRT). Evaluate cognitive function of patients with Wechsler adult intelligence scale-Chinese revised (WAIS-CR). HC were segmented into HC head (HH), HC body (HB) and HC tail (HT) and the indexes were then analyzed. WAIS-CR was tested before and 3months after radiotherapy. The mean radiation dose of left and right HC was (1147 ± 976)cGy, (1011 ± 602)cGy respectively. The radiation dose and the volume exposed in different dose of HH, HB and HT decreased in turn. For 5 patients, before and after radiotherapy, the regular-order score was 8.60 ± 1.34, 8.0 ± 1.00 (P = 0.43), while the reverse-order score was 5.80 ± 0.84, 5.20 ± 0.84 (P = 0.07). The radiation dose of HH, HB and HT was different, and the radiation dose of HH was the highest, which should be emphasized especially.
Introduction
Nasopharyngeal carcinoma (NPC) is common in Southern China. Intensity modulated radiotherapy (IMRT) is frequently used in NPC radiotherapy. Compared with two-dimensional radiotherapy and three-dimensional conformal radiotherapy, there is obvious dosimetric advantages in IMRT. While the target of tumor is covered highly conformal, the exposed dose of the organs at risk (OARs) minimize as low as possible. However the normal tissues surrounding the target that has not been concerned may be irradiated with relatively a lot of dose1. Hippocampus (HC) is closely related with intelligence and memory, and receives relatively a lot of radiation dose in the treatment of NPC patients2. The result of RTOG 09333 suggests that HC sparing during brain radiotherapy can alleviate memory impairment.
HC can be segmented into three parts: head (HH), body (HB) and tail (HT) according to anatomic structure. These three parts play different roles in Alzheimer’s disease4,5. At present, there is no study focusing on the radiation dose of the three parts of HC in NPC. This study makes a preliminary analysis of the radiation dose of the three parts.
Material and Methods
Patient clinical data
10 NPC patients treated from August 2015 to July 2016 in Zhejiang Cancer Hospital were enrolled. All patients were pathologically confirmed as NPC, 6 male cases, 4 female cases, 22–57 years old. According to the UICC 2010 stage of nasopharyngeal carcinoma, stage of the patients was T3-4N1-3M0, III-IVB. All 10 patients received 2–3 cycles of TP or PF (docetaxel + cisplatin/nadeplatin, fluorouracil + cisplatin/nadeplatin) induction chemotherapy, followed by IMRT and cisplatin/nadeplatin concurrent chemotherapy. Informed consent forms were signed by all patients. The ethics institutional review board of Zhejiang Cancer Hospital approved the protocols for data collection and analyses. All the methods described here were performed in accordance with the relevant guidelines and regulations.
Immobilization and scan
The patients were immobilized by head neck shoulder thermoplastic mask at comfortable position. CT-sim scan was completed with Philips Brilliance CT or GE Light Speed, and scan layer thickness was 3 mm. After CT-sim scan, MRI scan was obtained in the same posture and immobilization mode with SIEMENS Verio 3.0 T. The parameters of MRI scan were as follow: layer thickness 1.5 mm, layer space 0.45 mm, field of view (FOV) = 230 × 230 mm, TR 1440 ms, TE 11 ms, flip angle (FA) 150 degree, scan matrix 320 × 320, the rotation angle 90 degree, voxel size = 1.0 × 0.7 × 1.5 mm3. CT-sim and MRI scan images were transmitted into RayStation4.0 v (RaySearch Laboratories AB), then the CT and MRI images were fused and reconstructed for target and OARs delineation.
Target delineation and dose constraint
Target definition and prescription dose of nasopharynx and neck
PGTVnx + rn was the irradiated target of primary nasopharyngeal tumor and retropharyngeal lymph node, prescription dose 7040 cGy/32 F. PTV1 was the irradiated target of high-risk area, prescription dose 6080 cGy/32 F. PTV2 was the irradiated target of low-risk area including the neck lymphatic drainage area, prescription dose of 5440 cGy/32 F. PTVnd was the irradiated target of cervical lymph node, prescription dose 6400 cGy/32 F.
Dose constraint
At least 95% PTV was covered by the prescription dose. The volume of PTV receiving more than 110% of the prescription dose did not exceed 20%, and that receiving less than 93% of the prescription dose did not exceed 1%. Dose constraint of OARs was as follow: irradiated dose of brain stem less than 54 Gy; the maximum irradiated dose of spinal cord less than 45 Gy; dose constraint of other OARs see in expert consensus. HC was not listed as OAR in treatment plan, and there was no dose constraint for HC in the process of planning.
The delineation of HC and the segmentation of HH, HB and HT
The delineation of HC: According to criterion of RTOG 09336, bilateral HC(HC-L, HC-R) was delineated on T1 weighted MRI images in 10 patients by two established physicians. And all delineated HC were reviewed and revised by the same senior physician.
The definition of HH, HB and HT: According to the anterior to posterior direction, HC was segmented into three parts: HH, HB and HT by the ratio of 35%, 45% and 20% respectively7.
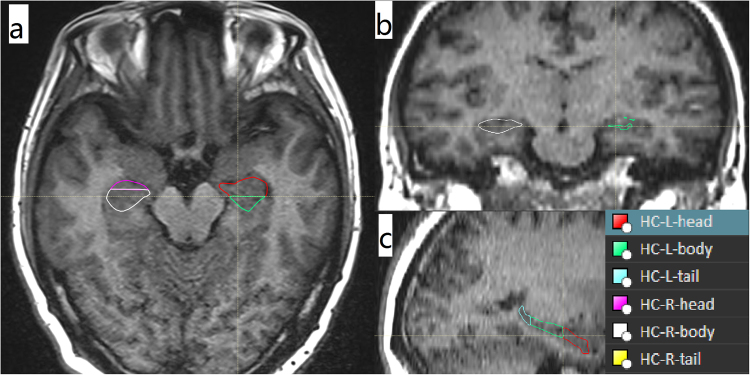
The segmentation of HH, HB and HT: Fixed an image on sagittal section in which HC showed, then confirmed the total layers of the HC that showed on coronal section. With the ratio of 35%, 45% and 20%, the respective total layers of HH, HB and HT was confirmed. Meanwhile, the two sagittal layers that were based on to divide HH, HB and HT were confirmed. The two sagittal layers were showed as two lines on transverse section. Based on the two lines, HC was segmented into HH, HB and HT layer by layer. The schematic diagram was showed in Figs 1 and 2.
Figure 1.
The segmentation of HC.
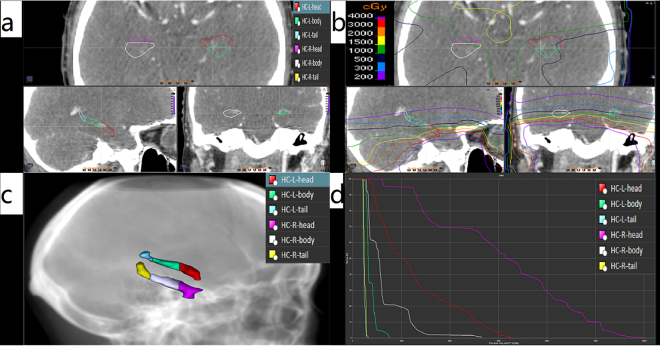
Figure 2.
The segmentation of HC and dose distribution. (a) The segmentation on two dimension; (b) The isodose curve on two dimension; (c) The segmentation on three dimension; (d) The DVH curve.
Dose parameters setting
Plan designation was completed with RayStation, and then the dose parameters were collected. Dn% was the radiation dose of n% volume of the target organ. D1% represented the maximum dose; D99% represented the minimum dose; Dmean represented the average dose. Vn% represented the percentage volume of target organ that was irradiated by nGy. All data were expressed as mean ± standard deviation.
Memory test
Wechsler adult intelligence scale-Chinese revised (WAIS-CR) was tested before and 3months after radiotherapy. All tests were carried out in quiet environment. The scale test was performed by specific physician. Only the digit span test of the WAIS-CR was used. Regular-order and reverse-order score were listed separately.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS, Chicago, IL) software package, version 18.0, for Windows. Single factor analysis of variance and paired T test were performed. A two-tailed P-Value less than 0.050 was considered as statistical significance in this study.
Results
All 10 patients received 2–3 cycles induction chemotherapy. Effects were evaluated as PR in 9 patients (nasopharyngeal lesions close to CR in 3 patients), SD in 1 patient. Then radiotherapy and concurrent chemotherapy followed.
There was relatively a lot of radiation dose in bilateral HC (showed in Table 1). D1%, D50%, Dmean and D99% of HH, HB and HT decreased in turn. The radiation dose of HH was much higher than that of HB and HT (showed in Table 2).
The volume that was irradiated with different dose of HH, HB and HT was different. V5–V50% decreased in turn (except for the V20% of HC-R). The volume of HH was much higher than that of HB and HT (showed in Table 3).
Five patients completed WAIS-CR test. Before and after radiotherapy, the mean value of regular-order scores was 8.60 ± 1.34, 8.0 ± 1.00 (P = 0.43), and the mean value of reverse-order scores was 5.80 ± 0.84, 5.20 ± 0.84 (P = 0.07).
Table 1.
The radiation dose of bilateral HC(cGy).
| Parameters | D1% | D50% | Dmean | D99% |
|---|---|---|---|---|
| Position | ||||
| HC-L | 3049 ± 1683 | 935 ± 972 | 1147 ± 976 | 396 ± 480 |
| HC-R | 2923 ± 1358 | 832 ± 581 | 1011 ± 602 | 389 ± 403 |
Table 2.
The radiation dose of HH, HB and HT.
| Position | HC-L | HC-R | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Exposed dose (cGy) | overall P value | P value between groups | Exposed dose (cGy) | overall P value | P value between groups | |
| HH | 3160 ± 1731 | 0.015* | 3121 ± 1410 | 0.001* | |||
| D1% | HB | 1546 ± 1334 | 0.003 | 0.001† | 1391 ± 793 | 0.000 | 0.000† |
| HT | 843 ± 1017 | 0.268‡ | 679 ± 714 | 0.131‡ | |||
| HH | 1682 ± 1363 | 0.081* | 1577 ± 991 | 0.013* | |||
| D50% | HB | 839 ± 979 | 0.052 | 0.019† | 713 ± 471 | 0.008 | 0.004† |
| HT | 526 ± 655 | 0.507‡ | 533 ± 627 | 0.586‡ | |||
| HH | 1739 ± 1317 | 0.076* | 1691 ± 942 | 0.006* | |||
| Dmean | HB | 890 ± 982 | 0.042 | 0.015† | 744 ± 483 | 0.002 | 0.001† |
| HT | 547 ± 688 | 0.461‡ | 531 ± 603 | 0.503‡ | |||
| HH | 902 ± 1016 | 0.288* | 833 ± 575 | 0.067* | |||
| D99% | HB | 533 ± 700 | 0.305 | 0.137† | 438 ± 384 | 0.082 | 0.042† |
| HT | 380 ± 465 | 0.657‡ | 391 ± 407 | 0.822‡ | |||
*Represents comparison between HH and HB; †Represents between HH and HT; ‡Represents between HB and HT.
Table 3.
The volume irradiated with different radiation dose of HH, HB and HT.
| Position | HC-L | HC-R | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Volume | P value | volume | P value | |||
| overall P value | P value between groups | overall P value | P value between groups | ||||
| HH | 80.01 ± 32.02 | 0.109* | 81.41 ± 30.97 | 0.120* | |||
| V5% | HB | 51.19 ± 44.41 | 0.011 | 0.003† | 54.24 ± 39.83 | 0.005 | 0.001† |
| HT | 22.88 ± 39.35 | 0.116‡ | 20.57 ± 41.90 | 0.057‡ | |||
| HH | 62.80 ± 36.20 | 0.020* | 66.52 ± 40.02 | 0.034* | |||
| V10% | HB | 24.75 ± 35.30 | 0.007 | 0.003† | 27.48 ± 37.25 | 0.026 | 0.012† |
| HT | 11.77 ± 31.50 | 0.406‡ | 19.07 ± 40.26 | 0.635‡ | |||
| HH | 44.53 ± 37.81 | 0.041* | 47.86 ± 39.41 | 0.021* | |||
| V15% | HB | 12.22 ± 31.13 | 0.052 | 0.030† | 12.48 ± 27.89 | 0.023 | 0.014† |
| HT | 10.00 ± 31.62 | 0.884‡ | 9.79 ± 28.09 | 0.853‡ | |||
| HH | 31.73 ± 33.89 | 0.120* | 37.38 ± 36.48 | 0.003* | |||
| V20% | HB | 10.33 ± 31.51 | 0.154 | 0.077† | 2.50 ± 5.97 | 0.005 | 0.006† |
| HT | 7.21 ± 22.80 | 0.817‡ | 5.65 ± 17.87 | 0.768‡ | |||
| HH | 16.31 ± 32.84 | 0.539* | 12.92 ± 17.23 | — | |||
| V30% | HB | 9.18 ± 29.04 | 0.472 | 0.225† | 0.00 ± 0.00 | — | — |
| HT | 2.09 ± 6.62 | 0.541‡ | 0.00 ± 0.00 | — | |||
| HH | 11.18 ± 27.74 | 0.180* | 3.05 ± 9.12 | — | |||
| V40% | HB | 1.22 ± 3.87 | 0.255 | — | 0.00 ± 0.00 | — | — |
| HT | 0.00 ± 0.00 | — | 0.00 ± 0.00 | — | |||
| HH | 4.70 ± 12.83 | 0.171* | 1.01 ± 3.20 | — | |||
| V50% | HB | 0.04 ± 0.13 | 0.281 | — | 0.00 ± 0.00 | — | — |
| HT | 0.00 ± 0.00 | — | 0.00 ± 0.00 | — | |||
Blod number indicated the radiation dose of HT was greater than HB; *Represents comparison between HH and HB; †Represents between HH and HT; ‡Represents between HB and HT; There is no statistic analysis when mean value was 0.
Discussion
The 5 year overall survival(OS) of stage I, II, III and IVa-b NPC patients was 100%, 94.3%,83.6% and 70.5% respectively8. Because of so good curative effect, it is extremely important to reduce complications and improve quality of life of NPC patients. Radical radiotherapy is the main treatment for non metastatic NPC patients. The intelligence, memory and cognitive function of NPC patients decreased after radiotherapy9,10. In this group, 5 patients completed the WAIS-CR test. After radiotherapy, the patients decreased in the mean value of regular-order and reverse-order repetition, and the P value of reverse-order repetition was close to statistical significance (P = 0.07). This result also indicated that cognitive function impairment decreased after radiotherapy in NPC patients.
Located in the inner side of temporal horn, HC is closely related to cognitive function. In 1957, Scoville and Milner11 reported that the removal of HC could cause memory impairment in space and time, especially the loss of ability to form new declarative long-term memory. The severity of impairment depended on the extension of resection degree of HC. Animal experiments showed that HC played an important role in cognitive impairment caused by radiotherapy. The granular cells of the HC dentate gyrus were extremely sensitive to radiotherapy, and only 1 Gy could reduce proliferation of the cells. The neurogenesis of HC was inhibited after radiotherapy, which was closely related to cognitive impairment12. The mice whose HC was irradiated with 10 Gy showed cognitive impairment in Barnes maze13. RTOG 0933 showed that HC sparing could reduce the cognitive impairment in patients who underwent brain radiotherapy3. Based on RTOG0933 protocol, animal experiments also demonstrated that the cognitive impairment test of the HC sparing mice was significantly better than that of non HC sparing group14. Cognitive impairment aggravated significantly when the D40% of bilateral HC was greater than 7.3 Gy in study of brain tumor15.
These results suggest that HC plays an important role in the cognitive impairment caused by radiotherapy. At present, whether Chinese 2010 nasopharyngeal carcinoma intensity modulated target expert consensus16 or RTOG 061517, HC was not listed as OAR, and the tolerance dose of HC is not clear.
The radiation dose of HC was reported differently under the technology of IMRT without HC sparing. Khodayari et al.2 reported that the Dmean of HC was 3027 cGy (range 1908-4799cGy), while the Dmean of HC was 1518 cGy reported by Gu et al.18. And the Dmean was (2411 ± 239)cGy in the report of Han et al.19. In this study, the Dmean of HC-L and HC-R was (1147 ± 976) cGy, (1011 ± 602) cGy respectively, which was lower than those reported previously. The possible reasons were as follow: on one hand there was certain randomness in the radiation dose of HC as there was no dose constraint; on the other hand patients in this group all underwent 2–3 cycles of induction chemotherapy. Then the irradiated target volume reduced, so the radiation dose of HC reduced to some extent.
HC is a symmetry structure that goes along from anterior-inferior to posterior-superior, and can be segmented into head, body and tail. In 2002 Hackert et al.7 segmented HC into HH, HB and HT with the ratio of 35%, 45% and 20% respectively, and did related research. Study showed that dysfunction of HH played an important role in memory impairment in patients with amnesia20. Oral memory deficits were associated with left HH atrophy in the patients with traumatic brain injury21. Yakushev et al.4 found that an increase in the diffusion rate of the left HH was significantly associated with delayed verbal memory recall test (DVR), and volume decrease in the left HB and HT was associated with DVR. In the study of Alzheimer’s disease, the atrophy of the HH, HB and HT was different, and the HH, HB and HT had unique relationship with the neurocognitive function and the indexes of cerebrospinal fluid5.
In view of different function of HH, HB and HT, we hypothesize that the role of HH, HB and HT may be different in neurocognitive impairment caused by radiotherapy. Therefore, it is necessary to analyze the radiation dose of HH, HB and HT.
The radiation dose was significantly different in HH, HB and HT. The D1%, D50%, Dmean and D99% of HH, HB and HT decreased in turn, and the radiation dose of HH was much higher than that of HB and HT. In Fig. 2b and d, the isodose curve also showed the dose difference of HH, HB and HT. There was no overall statistic difference in D99% of bilateral HC, D50% of HC-L, but there was overall statistic difference in all other parameters. Comparative analysis among HH, HB and HT showed that in comparison between HH and HB, there was statistic difference in D1%, D50%, Dmean of HC-R, D1% of HC-L; in comparison between HH and HT, all parameters reached statistic difference except for D99% of HC-L; as for the comparison between HB and HT, there was no statistic difference in D1%, D50%, Dmean, D99%. These results indicated that the differences between HH and HT, HH and HB, HB and HT decreased in turn. There was no statistic difference in some parameters, but the difference in mean value was obvious. The difference was not statistically significant possibly because of the small sample size.
The volume irradiated with different radiation dose of HH, HB and HT was different, and the volume of HH was much higher than that of the HB and HT. The V5%–V50% of HH, HB and HT decreased in turn (except for the V20% of HC-R). There was overall statistic difference in V5% and V10% of HC-L, V5% to V20% of HC-R, and there was almost statistic difference in V15% of HC-L (p = 0.052). Comparative analysis among HH, HB and HT showed that in comparison between HH and HB, there was statistic difference in V10% and V15% of the HC-L, and V10%–V20% of HC-R; in comparison between HH and HT, there was statistic difference in V5%–V15% of HC-L and V5%–V20% of HC-R; as for comparison between HB and HT, there was no statistic difference in all parameters of bilateral HC. From the beginning of V30%, the HB and HT of HC-R was 0, while V40% of HT-L was 0. The volume irradiated with different dose also showed that the difference between the HH and HT, HH and HB, HB and HT decreased in turn. There was no statistic difference in some parameters, but the difference in mean value was also obvious. The difference was not statistically significant possibly because of the small sample size.
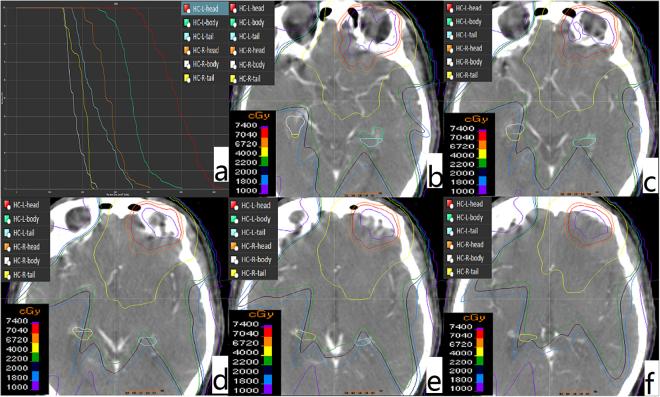
It is worth noting that the value of HT was greater than HB in V20% of HC-R (showed in Table 3). By rechecking the data, we found that there were 2 patients whose radiation dose of HT was greater than that of HB in HC-R. The first patient had extensive invasion lesions involving the skull base, left cavernous sinus, left orbital apex, left optic nerve and left internal and external rectus, and the dose curve was showed in Fig. 3. The other patient was just clivus invasion. This situation may occur randomly because of no HC sparing in the process of radiotherapy planning. It can be seen that because of different extension range, there is possibility that the radiation dose of HT is greater than HB.
Figure 3.
Diagram of the radiation dose of right HT was higher than HB. (a) The DVH curve; (b–f) the dose coverage of right HB and HT.
IMRT can make HC sparing plan, Han et al.19 reported that Dmean of HC could decreased from (2411 ± 239) cGy to (1414 ± 159) cGy in the IMRT sparing plan. Gu et al.18 reported that Dmean of HC could decrease from 1518 cGy to 899 cGy and 896 cGy respectively under technology of VMRT and IMRT sparing plan. Our results showed that under no HC sparing plan, the radiation dose of HH, HB and HT decreased in turn. We can infer that the radiation dose of the HH, HB and HT will also decrease in HC sparing plan. Compared with HB and HT, HH is closer to positions such as skull base, cavernous sinus etc that was easily invaded in locally advanced NPC. Therefore special attention should be paid to HH that was irradiated with higher dose.
Because of the anatomical characteristics of HC, the anterior-inferior part of HC is close to nasopharynx, while the posterior-superior part of HC is away from nasopharynx. It can be assumed that the radiation dose of HH, HB and HT was different. It is relatively difficult to segment HC into HH, HB and HT, and some method is difficult to implement22. This small sample study adopted a simple method which was relatively easy to complete the segmentation. As far as we know, we described the radiation dose of HH, HB and HT for the first time. We confirmed that the radiation dose of HH, HB and HT was different, and the radiation dose of HH, HB and HT decreased in turn. In future we can find more reliable and operable demarcation method, and expand the sample size for further dose study. Further improve the cognitive evaluation of neurological function and explore the roles of HH, HB and HT in cognitive function damage caused by radiotherapy.
Acknowledgements
This work was supported by Medical and Health Science and Technology Plan Project of Zhejiang Province Medical and Health Science and Technology Plan Project of Zhejiang Province [2016KYA041]; Natural Science Found of Zhejiang Province [LY16H160037]; National Natural Science Foundation of China [81672971].
Author Contributions
Chen Yuan-yuan, Chen Ming, Hu Qiao-ying and Chen Xiao-zhong conceived and designed the experiments. Sun Zong-wen, Shi lei, Xie Tie-ming and Hua Yong-hong performed the experiments. Sun Zong-wen, Kong yue and Du feng-le analyzed the data. Sun Zong-wen wrote the paper and prepared Figs 1 to 3. Li Qinglin revised the manuscript. All authors have reviewed the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Sun Zong-wen and Shi lei contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenthal DI, et al. Beam Path Toxicities to Non-Target Structures During Intensity-Modulated Radiation Therapy for Head and NeckCancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khodayari B, et al. Evaluation of hippocampus dose for patients undergoing intensity-modulated radiotherapy for nasopharyngeal carcinoma. The British Journal of Radiology. 2014;87:20130474. doi: 10.1259/bjr.20130474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gondi V, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yakushev I, et al. Increased hippocampal head diffusivity predicts impaired episodic memory performance in early Alzheimer’s disease. Neuropsychologia. 2010;48:1447–1453. doi: 10.1016/j.neuropsychologia.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Killiany RJ. Hippocampal Subregions are Differentially Affected in the Progression to Alzheimer’s Disease. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:132–140. doi: 10.1002/ar.21493. [DOI] [PubMed] [Google Scholar]
- 6.Gondi, V., Tome, W. A., Rowley, H. A. & Mehta, M. P. Hippocampal Contouring: A Contouring Atlas for RTOG 0933, https://www.rtog.org/CoreLab/ContouringAtlases/HippocampalSparing.aspx (2014).
- 7.Hackert VH, et al. Hippocampal Head Size Associated with Verbal Memory Performance in Nondemented Elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Lee PW, Hung BK, Woo EK, Tai PT, Choi DT. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488–492. doi: 10.1136/jnnp.52.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao K, et al. Cognitive Function Before and After Intensity-Modulated Radiation Therapy in Patients With Nasopharyngeal Carcinoma: A Prospective Study. International Journal of Radiation Oncology*Biology*Physics. 2010;77:722–726. doi: 10.1016/j.ijrobp.2009.06.080. [DOI] [PubMed] [Google Scholar]
- 11.SCOVILLE WB, MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizumatsu S, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 13.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/RR3206. [DOI] [PubMed] [Google Scholar]
- 14.Tomé WA, et al. A mouse model replicating hippocampal sparing cranial irradiation in humans: A tool for identifying new strategies to limit neurocognitive decline. Sci. Rep. 2015;5:14384. doi: 10.1038/srep14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal Dosimetry Predicts Neurocognitive Function Impairment After Fractionated Stereotactic Radiotherapy for Benign or Low-Grade Adult Brain Tumors. International Journal of Radiation Oncology*Biology*Physics. 2013;85:348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Committee on clinical staging of nasopharyngeal carcinoma in China Expert consensus on target area and dose design guidelines for 2010 nasopharyngeal carcinoma patients with radiotherapy. Chin J Radiat Oncol. 2011;20:267–269. [Google Scholar]
- 17.Lee NY, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu WD, Li QL, Tian Y, Mo JC, Pei HL. Effects of hippocampal-sparing intensity-modulated radiotherapy on dose distribution of target volume and organs at risk in locally advanced nasopharyngeal carcinoma. Chin J Radiat Oncol. 2017;26:6–11. [Google Scholar]
- 19.Han G, et al. Evaluation of the dosimetric feasibility of hippocampal sparing intensity-modulated radiotherapy in patients with locally advanced nasopharyngeal carcinoma. Plos One. 2014;9:e90007. doi: 10.1371/journal.pone.0090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouchi Y, et al. Altered glucose metabolism in the hippocampal head in memory impairment. Neurology. 1998;51:136–142. doi: 10.1212/WNL.51.1.136. [DOI] [PubMed] [Google Scholar]
- 21.Ariza M, et al. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty AM, Yu Q, Flinn R, Ofen N. A reliable and valid method for manual demarcation of hippocampal head, body, and tail. Int J Dev Neurosci. 2015;41:115–122. doi: 10.1016/j.ijdevneu.2015.02.001. [DOI] [PubMed] [Google Scholar]