FIG 2 .
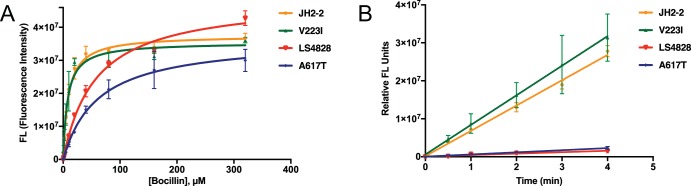
Comparative affinities of PBP4 from JH2-2 and LS4828 and variants thereof. LS4828 PBP4 and the A617T variant show reduced Bocillin FL affinities and apparent acylation rates. (A) Michaelis-Menten (Km) curves were derived from the Bocillin FL binding of each PBP4 enzyme by using SDS-PAGE data and graphing relative fluorescence intensity versus the substrate concentration. Error bars indicate the standard deviation of the mean of two independent experiments. (B) Apparent rates of 10 µM Bocillin FL acylation over time are graphically presented for all PBP4 variants with error bars indicating the standard deviation of the mean of two independent experiments. The slopes of these progress curves were determined in GraphPad Prism version 5 and represent the rate of formation of the stable PBP-Bocillin FL (acyl) complex. The A617T and LS4828 PBP4-Bocillin FL complexes are formed at similar lower rates than those formed by JH2-2 and V223I.