Key Points
Question
What is the association between reperfusion and brain edema inpatients with acute ischemic stroke?
Findings
In this exploratory post hoc analysis of the MR CLEAN trial, successful reperfusion reduced the odds of brain edema, measured by midline shift, by 73%. Reducing midline shift also mediated part of the favorable 90-day neurological outcome.
Meaning
Reperfusion was associated with reduced brain edema in patients with acute ischemic stroke enrolled in the MR CLEAN trial.
Abstract
Importance
It is uncertain whether therapeutic reperfusion with endovascular treatment yields more or less brain edema.
Objective
To elucidate the association between reperfusion and brain edema. The secondary objectives were to evaluate whether brain edema could partially be responsible for worse outcomes in patients with later reperfusion or lower Alberta Stroke Program Early Computed Tomography Score.
Design, Setting, and Participants
This was a post hoc analysis of the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN), which was a prospective, randomized, multicenter clinical trial of endovascular treatment compared with conventional care of patients with acute anterior circulation ischemic stroke. Of 502 patients enrolled from December 2010 to June 2014, 2 patients declined to participate. Additionally, exclusion criteria were absence of follow-up imaging or presence of parenchymal hematoma, resulting in 462 patients included in this study. Brain edema was assessed retrospectively, from December 10, 2016, to July 24, 2017, by measuring midline shift (MLS) in all available follow-up scans. Observers were blinded to clinical data.
Main Outcomes and Measures
Midline shift was assessed as present or absent and as a continuous variable. Reperfusion status was assessed by the modified thrombolysis in cerebral infarction score in the endovascular treatment arm. The modified arterial occlusive lesion score was used to evaluate the recanalization status in both arms. The modified Rankin scale score at 90 days was used for functional outcome.
Results
Of 462 patients, the mean (SD) age was 65 (11) years, and 41.8% (n = 193) were women. Successful reperfusion and recanalization were associated with a reduced likelihood of having MLS (adjusted common odds ratio, 0.25; 95% CI, 0.12-0.53; P < .001 and adjusted common odds ratio, 0.34; 95% CI, 0.21-0.55; P < .001, respectively). Midline shift was partially responsible for worse modified Rankin scale scores in patients without reperfusion or recanalization (MLS changed the logistic regression coefficients by 30.3% and 12.6%, respectively). In patients with delayed reperfusion or lower Alberta Stroke Program Early Computed Tomography Score, MLS mediated part of the worse modified Rankin scale scores, corresponding to a change in the regression coefficient of 33.3% and 64.2%, respectively.
Conclusions and Relevance
Successful reperfusion was associated with reduced MLS. This study identifies an additional benefit of reperfusion in relation to edema, as well as rescuing ischemic brain tissue at risk for infarction.
Trial Registration
Netherlands Trial Registry number: NTR1804 and Current Controlled Trials number: ISRCTN10888758
This study examines the association between reperfusion and brain edema in patients with acute ischemic stroke.
Introduction
Therapeutic reperfusion with endovascular treatment (EVT) is consistently associated with better long-term functional outcome in anterior circulation acute ischemic stroke.1 Reperfusion may act in part by arresting infarct growth and rescuing ischemic tissue at risk.2,3,4 Given the demonstrated benefit in patients with large vessel occlusion, identifying additional patients who may benefit from reperfusion therapy is critical. Extending the time by carefully selecting small core lesions shows substantial promise.5 However, the role for treating patients with lower Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is uncertain. Understanding the mechanisms that mediate or limit the benefits of reperfusion in these contexts may help refine appropriate patient selection.
Reperfusion injury,6,7,8 and specifically reperfusion edema, may reduce the benefit of thrombectomy. However, studies on the association between reperfusion and brain edema have yielded conflicting results. Findings from rodent and primate animal studies suggest that reperfusion can augment the development of edema.9,10,11 Conversely, some investigations have indicated that successful reperfusion may attenuate edema.12,13 Additional studies have shown that persistent occlusion is associated with a greater risk of malignant edema.14,15 However, to our knowledge, none have included EVT cohorts with highly effective thrombectomy devices.
The successes of EVT for ischemic stroke have highlighted the salience of clarifying the association between reperfusion and edema in patients with stroke. The goal of this analysis was to evaluate the association between reperfusion status and brain edema in patients enrolled in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) cohort.16,17 Based on preliminary data,12 our hypothesis was that the beneficial effect of reperfusion and recanalization on outcome may be mediated in part by a reduction in edema. A secondary hypothesis was that in patients with delayed reperfusion or lower ASPECTS, brain edema could partially be responsible for the worse functional outcome.
Methods
Patient Characteristics
Patient imaging and clinical data were retrospectively selected from the MR CLEAN cohort.16,17 The MR CLEAN study was a prospective, randomized, multicenter clinical trial of patients with acute ischemic stroke with a National Institutes of Health Stroke Scale score of 2 or more, a relevant proximal intracranial arterial occlusion of the anterior circulation confirmed by neuroimaging, and the ability to start EVT within 6 hours after stroke onset. The original study randomized patients to EVT plus conventional care compared with conventional care only. Detailed inclusion and exclusion criteria have been reported previously.16,17 Written informed consent was obtained from all patients or their legally authorized representatives. The original study protocol was approved by a central medical ethics committee and the research board of each participating center. In the original trial, 2 patients declined participation immediately after randomization.16,17
Additional exclusion criteria for this exploratory post hoc analysis were the absence of follow-up (FU) imaging and the presence of parenchymal hematoma types 1 and 2 on FU computed tomography (CT) imaging.18 Posthemicraniectomy patients were not excluded in our study because hemicraniectomy procedures were usually performed after the 24-hour FU scan. In these cases, midline shift (MLS) was measured in the early FU scan before the surgical procedure.
Imaging Analysis and Outcomes
The imaging acquisition protocol was based on the MR CLEAN trial, as described previously,16,17 and included a baseline noncontrast CT and CT angiogram (CTA) (n = 462), a follow-up CTA or magnetic resonance angiogram at 24 hours (n = 440), and a second follow-up noncontrast CT or magnetic resonance imaging scan at day 5 to 7 (n = 352). The baseline infarct size was assessed by the ASPECTS, as previously described.19 Cerebral collateral vessels were assessed on the baseline CTA by the MR CLEAN imaging committee, using a 4-point scale category score (0, absent collaterals; 1, filling <50% of occluded area; 2, filling >50% but less <100%; or 3, filling 100% of occluded area).20
Midline shift was measured on all available FU scans at 24 hours and 5 to 7 days. These times were categorized as early FU (24 hours) and late FU (5-7 days), respectively. Midline shift measurements were performed by readers who were blinded to the clinical data. Midline shift was assessed as both a dichotomous (present or absent) and a continuous variable. Midline shift was evaluated both ways to ensure that the nonparametric analysis of the continuous variable was not affected by the skewness of the data. The presence of MLS was determined by 2 readers from the MR CLEAN imaging committee (interrater reliabilities of 0.87 and 0.81 on early FU and late FU scans assessments, respectively), and was defined as any deviation of midline structures (eg, the septum pellucidum). The quantitative assessment of MLS was performed on patients previously categorized as having MLS. It was measured in millimeters at the level of the septum pellucidum and was assessed by a neuroradiologist (B.G.D.) using previously published approaches (eFigure 1 in the Supplement).21,22,23
Recanalization was assessed on the 24-hour CTA in both treatment arms, using the modified arterial occlusive lesion score.24 Scores were dichotomized into successful (modified arterial occlusive lesion, 3; complete recanalization) and unsuccessful (modified arterial occlusive lesion <3). Reperfusion status was assessed on digital subtraction angiograms in the EVT arm using the modified thrombolysis in cerebral infarction (mTICI) score. The mTICI score was assigned following completion of the thrombectomy procedure. The mTICI score ranges from 0 (no antegrade reperfusion) to 3 (complete antegrade reperfusion, with absence of visualized occlusion in all distal branches), and successful reperfusion was defined as mTICI 2b or 3.24 The time from stroke onset to reperfusion was indirectly assessed by the time from onset stroke to the end of EVT (time to reperfusion), measured in minutes.
Neurological functional outcome at 90 days was assessed by the modified Rankin Scale (mRS). The mRS scale ranges from 0 (no residual stroke symptoms) to 6 (death) and was evaluated across the entire score range as an ordinal variable.
Statistical Analysis
The patients were originally enrolled from December 2010 to June 2014, and MLS was assessed retrospectively. Descriptive analysis of baseline variables, treatment, and outcome were reported for groups with presence or absence of MLS using the χ2 test for categorical data, the Mann-Whitney U test for nonnormally distributed continuous data, and the t test for normally distributed continuous data.
Associations of recanalization, reperfusion, and treatment arm (EVT or conventional) with MLS were assessed by binary logistic regression (for dichotomous MLS) and by Mann-Whitney U test (for continuous MLS). Association between time to reperfusion and MLS was assessed by binary logistic regression and linear regression for dichotomous MLS and continuous MLS, respectively. Unadjusted and adjusted analyses were performed in the binary logistic and linear regression models. To adjust for baseline prognostic variables, the models included age, sex, stroke severity (baseline National Institutes of Health Stroke Scale), time from stroke onset to randomization, history of diabetes mellitus, admission serum glucose, collateral score, baseline ASPECTS, atrial fibrillation, smoking, and prior stroke.
Univariable and multivariable ordinal logistic regression was used to assess the association between MLS and mRS at 90 days. The effect of MLS on outcome was expressed as an unadjusted and adjusted common odds ratios (cOR and acOR) for a shift in the direction of worse outcome on the mRS at 90 days.
Mediation analyses25,26 were performed to determine whether MLS influences the association of reperfusion, recanalization, time to reperfusion, and ASPECTS with functional outcome. Mediation analysis consists of a 4-step procedure detailed in eFigure 2 in the Supplement. The Sobel test was used to determine statistical significance of the mediation effect. The percent difference of the coefficients was measured after introducing MLS as the mediator. All statistical analyses were performed with IBM SPSS Statistics, version 24 software (SPSS Inc), and P less than .05 was considered statistically significant. All P values were 2-sided.
Results
Patient Characteristics
Of the 500 patients enrolled in the MR CLEAN trial, 38 patients were excluded owing to absence of FU imaging (n = 9) or presence of parenchymal hematoma (n = 29; 2 with parenchymal hematoma type 1 and 27 with parenchymal hematoma type 2). In total, 462 patients were included in our analysis, of which 46.8% (n = 216) had MLS present on any of the FU scans. The characteristics of patients with and without MLS are listed in the Table. In patients with MLS, there was a significantly higher baseline National Institutes of Health Stroke Scale, higher admission serum glucose, a lower ASPECTS, worse collateral score, longer time to reperfusion, a higher rate of internal carotid artery terminus arterial occlusion, a higher rate of hemicraniectomy, and worse 90-day mRS.
Table. Patient Characteristics of Those With and Without Midline Shift.
| Characteristics | With Midline Shift (n = 216) |
Without Midline Shift (n = 246) |
P Value |
|---|---|---|---|
| Age, median (IQR), y | 65 (53-77) | 66 (51.5-74.5) | .71 |
| Male, No. (%) | 126 (58.3) | 143 (58.1) | .96 |
| Time from onset to randomization, median (IQR), min | 222 (184.2-259.7) | 193.5 (141-246) | .08 |
| Admission NIHSS, median (IQR) | 19 (16-22) | 16 (13-19) | .001 |
| Baseline ASPECTS, median (IQR) | 8 (7-9) | 9 (8-10) | <.001 |
| Admission systolic blood pressure, median (IQR), mm Hg | 140 (123-157) | 145 (129.5-160.5) | .71 |
| Admission diastolic blood pressure, median (IQR), mm Hg | 80 (70-90) | 82 (74.5-89.5) | .77 |
| Admission serum glucose, median (IQR), mmol/L | 6.8 (6-7.9) | 6.5 (5.7-7.5) | .03 |
| Baseline medical history, No. (%) | |||
| Previous stroke | 22 (10.2) | 25 (10.2) | .99 |
| Diabetes mellitus | 31 (14.4) | 26 (10.6) | .22 |
| Atrial fibrillation | 64 (29.6) | 58 (23.6) | .14 |
| Smoke | 60 (27.8) | 76 (30.9) | .46 |
| Occlusion location on baseline CTA, No. (%) | |||
| ICA-T | 79 (36.6) | 53 (21.5) | .01 |
| M1 | 125 (57.9) | 165 (67.1) | |
| M2 | 10 (4.6) | 26 (10.6) | |
| A2 | 1 (0.5) | 2 (0.8) | |
| Collateral score, No. (%) | |||
| 0: Absent collaterals | 20 (9.3) | 4 (1.6) | <.001 |
| 1: Filling <50% of occluded area | 73 (34.1) | 48 (19.7) | |
| 2: >50% to <100% | 73 (34.1) | 113 (46.3) | |
| 3: 100% of occluded area | 48 (22.4) | 79 (32.4) | |
| Endovascular treatment, No. (%) | 89 (41.2) | 113 (45.9) | .31 |
| Successful recanalization status, No. (%) | 65 (38.9) | 134 (62.6) | <.001 |
| Successful reperfusion status, No. (%) | 37 (43.5) | 72 (72.7) | <.001 |
| Time to reperfusion, median (IQR) | 271.5 (229.5-313.5) | 252.5 (205-300) | .02 |
| Functional outcome (mRS), median (IQR) | 4 (3-5) | 2 (1-3) | <.001 |
| Hemicraniectomy, No. (%) | 22 (10.1) | 2 (0.9) | .001 |
Abbreviations: A2, anterior cerebral artery, segment 2; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CTA, computed tomography angiography; ICA-T, terminal internal carotid artery; IQR, interquartile range; M1, middle cerebral artery, segment 1; M2, middle cerebral artery, segment 2; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Factors Associated With Midline Shift
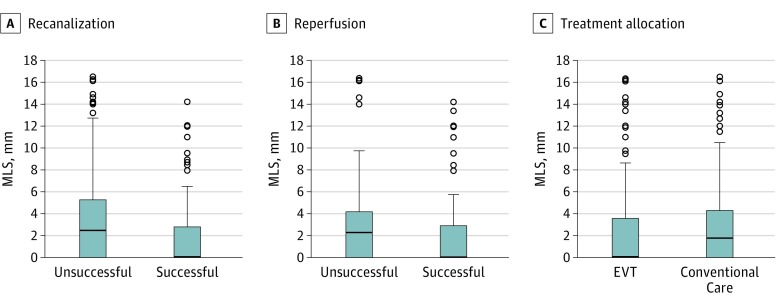
First, we evaluated the recanalization score on 24-hour CTA in both treatment arms. The modified arterial occlusive lesion score was available in 381 of 462 patients (82.5%). The rate of successful recanalization on 24-hour CTA was 34.7% in the conventional arm and 65.3% in the EVT arm. Patients with successful recanalization had a lower frequency of MLS (32.7% vs 56% in those with unsuccessful recanalization; P < .001). In binary logistic regression, successful recanalization was associated with a reduced likelihood of having MLS (cOR, 0.38; 95% CI, 0.25-0.56; Nagelkerke R2, 0.07; P < .001 and acOR, 0.34; 95% CI, 0.21-0.55; Nagelkerke R2, 0.20; P < .001). Accordingly, when MLS was evaluated as a continuous variable, patients with successful recanalization had a lower median MLS value on the late FU scan (median, 0 mm; IQR, 0-2.8 mm vs median, 2.4 mm; IQR, 0-5.3 mm; P < .001; Figure 1A) and early FU scan (median, 0 mm; IQR, 0-1.8 mm vs median, 0 mm; IQR, 0-3.3 mm; P = .04; eFigure 3 in the Supplement).
Figure 1. Midline Shift (MLS) on the Day 5 to 7 Computed Tomography (CT) Scan Based on Recanalization, Reperfusion, and Treatment Allocation.
A, MLS measurements based on recanalization status on follow-up CT angiogram. The box represents the interquartile range (25% to 75%), the solid horizontal line represents the median value, error bars encompass the 10th to 90th percentiles, small circles represent the outlier values, and P < .001. B, MLS values in relation to modified thrombolysis in cerebral infarction reperfusion status; P = .001. C, MLS values in relation to treatment allocation; P = .18. EVT indicates endovascular treatment.
We next evaluated reperfusion in the EVT arm (n = 202), using the mTICI score that was available in 91.1% of patients (n = 184). Successful reperfusion was observed in 109 of 184 patients (59.2%). Patients with successful reperfusion had a lower rate of MLS compared with the unsuccessful reperfusion group (33.9% vs 64%, P < .001). In binary logistic regression, successful reperfusion was associated with a lower likelihood of MLS in unadjusted and adjusted models (cOR, 0.29; 95% CI, 0.16-0.53; Nagelkerke R2, 0.11; P < .001 and acOR, 0.25; 95% CI, 0.12-0.53; Nagelkerke R2, 0.22; P < .001). The median MLS values were lower in the successful reperfusion group compared with unsuccessful reperfusion cases on the late FU (median, 0 mm; IQR, 0-2.9 mm vs median, 2.3 mm; IQR, 0-4.1 mm; P = .001, Figure 1B) and early FU scans (median, 0 mm; IQR, 0-2.2 mm vs median, 1.3 mm; IQR, 0-4.2 mm; P = .01; eFigure 3 in the Supplement). A representative example of a successful vs unsuccessful reperfusion is shown in Figure 2.
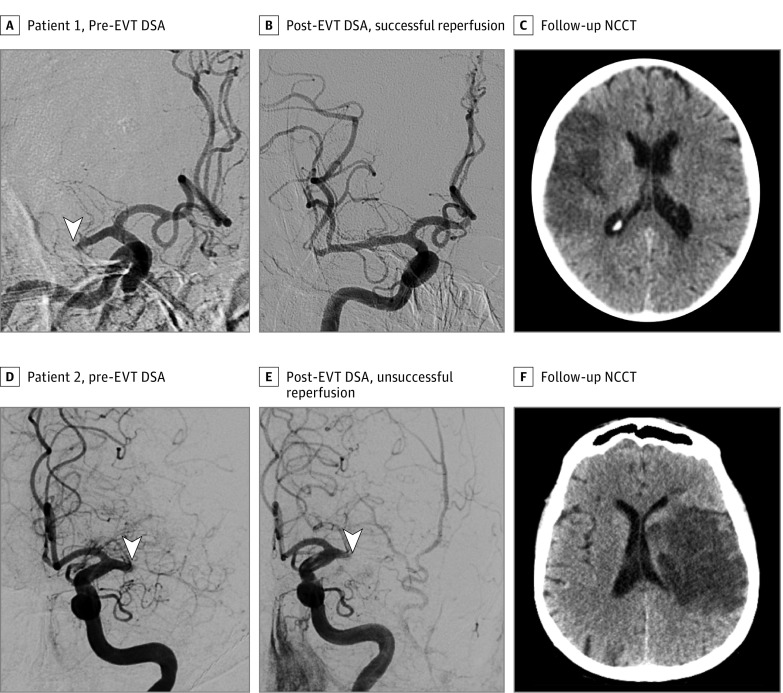
Figure 2. Examples of the Association Between Reperfusion Status and Midline Shift.
Digital subtraction angiogram (DSA), both prior to and after endovascular treatment (EVT), and the follow-up noncontrast computed tomography (NCCT) are illustrated. Patient 1 with successful reperfusion (A) shows a complete right M1 occlusion (arrowhead) seen on the pretreatment DSA frontal view. B, Post-EVT DSA shows successful reperfusion. C, Follow-up NCCT at day 5 shows a hypoattenuated area on the right frontoparietal lobe with a minor mass effect and lack of MLS. Patient 2 with unsuccessful reperfusion (D) had a complete left M1 occlusion (arrowhead) seen on pre-EVT DSA lateral view. E, No reperfusion (arrowhead) is observed on posttreatment DSA. F, The 24-hour follow-up NCCT shows a hypoattenuated area on the left frontoparietal lobe, corona radiata, and caudate nucleus with mass effect and a midline shift of 3 mm.
A longer time to reperfusion was associated with an increased likelihood of having MLS (cOR, 1.007; 95% CI, 1.003-1.011; Nagelkerke R2, 0.10; P < .001 and acOR, 1.012; 95% CI, 1.004-1.020; Nagelkerke R2, 0.20; P = .003). For each 90 minutes of delay in reperfusion, there was an increase in MLS of 1.0 mm and 0.80 mm on the early FU scans according to the unadjusted and adjusted analyses, respectively (β, .011; 95% CI, 0.005-0.017; P = .001 in unadjusted analysis and β, .008; 95% CI, 0.002-0.015; P = .002 in adjusted analysis) and of 1.4 mm and 1.24 mm on the late FU scans for both the unadjusted and adjusted models (β, .015; 95% CI, 0.008-0.023; P < .001 in unadjusted model and β, .014; 95% CI, 0.005-0.021; P = .002 in adjusted model).
We next examined the effect of treatment allocation on MLS. Midline shift was more frequently observed in the conventional arm (58.8%) than in the EVT arm (41.2%), but this was not statistically significant in unadjusted and adjusted binary logistic regression models (cOR, 0.82, 95% CI, 0.57-1.19; P = .31 and acOR, 0.78, 95% CI, 0.52-1.17; P = .27, respectively). Similarly, there were no statistically significant differences in the median MLS values between the treatment groups (Figure 1C and eFigure 3 in the Supplement), although a smaller MLS was observed in the EVT arm on the late FU scans (median, 0 mm; IQR, 0-3.6 mm for the EVT arm vs median, 1.7 mm; IQR, 0-4.3 mm for conventional care; P = .18).
Midline Shift and Functional Outcome
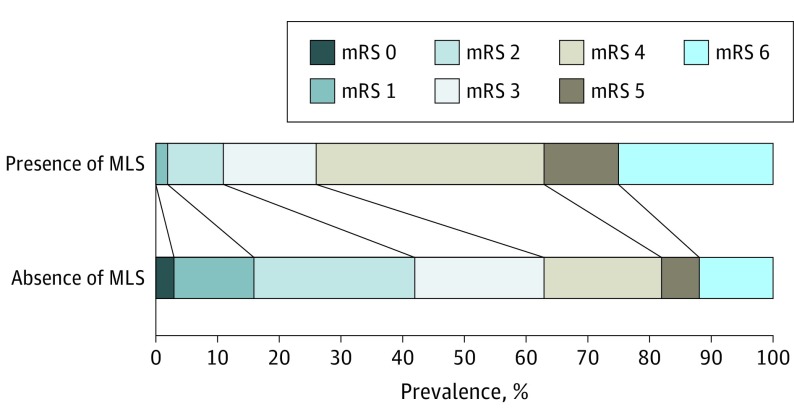
The presence of MLS was associated with a worse functional outcome (cOR, 4.22; 95% CI, 2.98-5.98; P < .001 and acOR, 3.49; 95% CI, 2.38-5.12; P < .001; Figure 3). There was a shift toward worse outcome, with a cOR of 1.21 for each millimeter increase in MLS in both early and late FU scans. In the adjusted analyses with continuous MLS values, the acORs were 1.24 (95% CI, 1.17-1.33) and 1.21 (95% CI, 1.15-1.30) in the early and late FU scans, respectively (P < .001, eTable in the Supplement).
Figure 3. The Modified Rankin Scale (mRS) Distribution for the Groups of Patients With and Without Midline Shift (MLS).
Scores range from 0 (no symptoms) to 6 (death). Black lines indicate shifts in mRS values across MLS groups (P < .001).
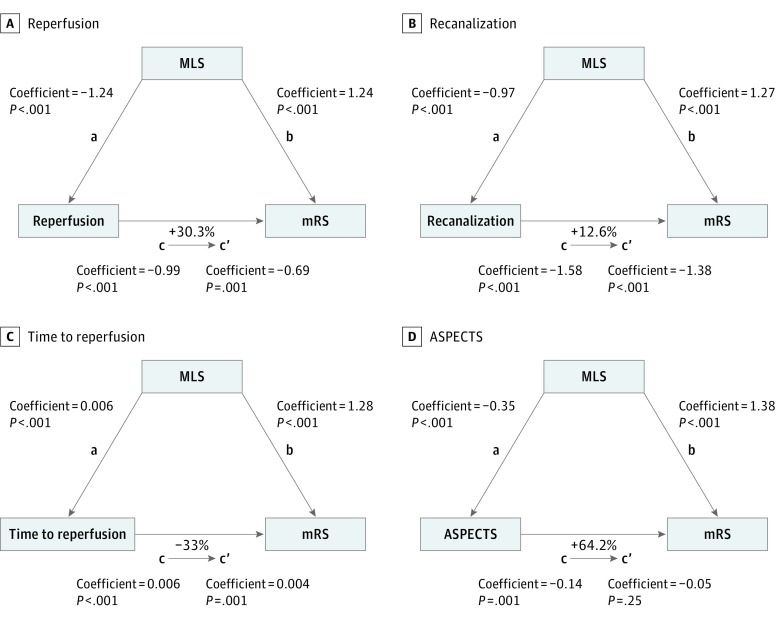
In the mediation analyses (Figure 4), after including MLS as a mediator, the ordinal regression coefficient was reduced by 30.3% in reperfusion (Figure 4A) and 12.6% in recanalization (Figure 4B), consistent with MLS partially mediating the association between recanalization or reperfusion with outcome. For the evaluation of time to reperfusion, the percentage difference of the coefficients between time to reperfusion and outcome was 33.3% (Figure 4C) after including MLS as a mediator. For ASPECTS, the mediation effect was stronger, and MLS was responsible to change 64.2% of the coefficient value (Figure 4D). For all the analyses, the Sobel test was statistically significant.
Figure 4. Mediation Analyses of Midline Shift (MLS) With Neurological Outcome.
Each step of the analyses evaluating the mediation effect of MLS on the association of reperfusion (A), recanalization (B), time to reperfusion (C) and Alberta Stroke Program Early Computed Tomography Score (ASPECTS) with the functional outcome (D; modified Rankin Scale [mRS] at 90 days) are illustrated. The coefficient and P values of the regression equations of each step (steps a, b, c, and c’) are described. The analyses were performed for a shift in the direction of worse functional outcome. The percentage differences of the coefficients (1−c’/c) are also shown.
Discussion
In this study, we investigated the association between reperfusion or recanalization and brain edema in the MR CLEAN trial. In patients with successful restoration of blood flow, defined by either recanalization or reperfusion measures, we consistently found less MLS. Later reperfusion and lower ASPECTS were related to increased MLS, suggesting that edema formation sufficient to cause MLS may limit the benefits of reperfusion in these patients. Moreover, we demonstrated that worse functional outcome associated with unsuccessful blood flow restoration, lower ASPECTS, or later reperfusion could be explained partially by brain edema formation as measured by MLS.
Prior studies have evaluated the association between reperfusion and brain edema in humans. Horsch et al27 found an increased odds of having brain edema in patients with unsuccessful recanalization, but the association was not statistically significant. Similarly, a lack of recanalization have been described as a predictor of malignant cerebral edema.13,28,29 In 2017, Irvine et al12 demonstrated an association between successful reperfusion and reduced edema, but this study did not include patients with current thrombectomy devices.12 In the context of these prior studies, our analysis provides additional support for the concept that reperfusion may have several benefits, not only by rescuing ischemic tissue at risk but also by reducing brain edema.
There is a well-established association between cerebral edema and poor outcome or death, particularly in severe stroke with malignant progression.14,15,28 Proximal arterial occlusion, a higher baseline National Institutes of Health Stroke Scale, higher baseline glucose,30 poor collaterals,31 and lower ASPECTS are associated with malignant brain edema.14,15,28,29,32 The observation that decompressive hemicraniectomy, if performed early enough,33,34 can improve outcome in a subset of patients supports the conclusion that edema formation has a more direct relation with poor outcome. Emerging data suggests that edema may also play a role in moderate stroke,21 although a causal association has not been established. The finding that MR CLEAN patients with MLS had worse outcomes further highlights the potential role of edema in moderate to severe stroke.
After the restoration of blood flow, the secondary goal of stroke clinical care is the mitigating factors that may exacerbate further neurological deterioration. For example, treating brain edema is an important approach in the subsequent acute care and includes options such as surgical decompression or osmotic therapy and potential preventive strategies.23,35 Our data could assist the selection of patients for brain edema prevention because in patients with lower ASPECTS or delayed reperfusion, worse outcome was partially explained by the MLS.
Limitations and Strengths
This study has several limitations. First, brain edema was assessed retrospectively by measuring MLS, which is an indirect measure of the mass effect rather than a direct assessment of water concentration. Approximately half of patients did not have measurable MLS but may have had a small amount of edema that did not lead to a change in the MLS measurement. Nevertheless, MLS is considered an easily assessable and reasonable quantitative parameter for brain edema in patients with acute ischemic stroke that has been linked to clinical outcome.36,37 Second, we excluded patients with parenchymal hemorrhage types 1 and 2, consistent with prior studies,21,22 although edema and hemorrhage frequently coexist. However, the number of patients excluded was small, and excluding them would bias toward the null hypothesis. Finally, the mediation analysis does not confirm a causal association of edema owing to the observational nature of the data.
The strengths of this study include the analysis of brain edema in a randomized, multicenter study design that evaluated highly effective thrombectomy devices. To our knowledge, this is also the first study to assess the mediation effect of MLS on functional outcome based on reperfusion and recanalization status, time to reperfusion, and ASPECTS in patients with acute ischemic stroke.
Taken together, our data provide insight into the association between reperfusion and brain edema. Our study reinforces the benefits of early reperfusion and recanalization in reducing brain edema and suggests that EVT does not increase the extension of brain edema, as has been reported in preclinical models.9,10,11 These data also suggest that edema may help explain the previous observation that final infarct volume only partially accounts for the EVT effect on outcome.38 The identification of predictors of brain edema in our data could help the selection of patients with higher risk of developing brain edema and could guide appropriate patient selection for edema prevention. Further prospective studies are warranted to confirm the potential causative role of reperfusion and especially treatment allocation in influencing brain edema in patients with acute ischemic stroke.
Conclusions
Successful reperfusion is associated with reduced mass effect, as measured by MLS. Midline shift was partially responsible for the worse mRS at 90 days in patients with unsuccessful blood flow restoration, lower ASPECTS, or later reperfusion. This study confirms a pleiotropic benefit of reperfusion in addition to rescuing ischemic brain tissue at risk for infarction.
eFigure 1. Method of Midline Shift Measurement.
eFigure 2. Steps of Mediation Analysis.
eFigure 3. Midline Shift Values on 24 Hour CT Scan (Early FU) According to (A) Reperfusion Status, (B) Recanalization Status, and (C) Treatment Allocation.
eTable. Common Odds Ratios (cOR) Expressing the Association between Continuous MLS and a Shift in the Direction of Worse Outcome on the mRS 90 Days.
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 2.Heiss WD, Huber M, Fink GR, et al. . Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab. 1992;12(2):193-203. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg MD, Pulsinelli WA. The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Ann Neurol. 1994;36(4):553-554. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Thijs VN, Wechsler L, et al. ; DEFUSE Investigators . Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508-517. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct [published online November 11, 2017]. N Engl J Med. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990;47(11):1245-1254. [DOI] [PubMed] [Google Scholar]
- 7.Kidwell CS, Saver JL, Starkman S, et al. . Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52(6):698-703. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Silva MD, Liu KF, et al. . Secondary decline in apparent diffusion coefficient and neurological outcomes after a short period of focal brain ischemia in rats. Ann Neurol. 2000;48(2):236-244. [PubMed] [Google Scholar]
- 9.Pillai DR, Dittmar MS, Baldaranov D, et al. . Cerebral ischemia-reperfusion injury in rats: a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29(11):1846-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell BA, Symon L, Branston NM. CBF and time thresholds for the formation of ischemic cerebral edema, and effect of reperfusion in baboons. J Neurosurg. 1985;62(1):31-41. [DOI] [PubMed] [Google Scholar]
- 11.Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol. 1997;147(2):353-360. [DOI] [PubMed] [Google Scholar]
- 12.Irvine HJ, Ostwaldt A-C, Bevers MB, et al. . Reperfusion after ischemic stroke is associated with reduced brain edema [published online July 21, 2017]. J Cereb Blood Flow Metab. 2017;X17720559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheripelli BK, Huang X, MacIsaac R, Muir KW. Interaction of recanalization, intracerebral hemorrhage, and cerebral edema after intravenous thrombolysis. Stroke. 2016;47(7):1761-1767. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309-315. [DOI] [PubMed] [Google Scholar]
- 15.Thomalla G, Hartmann F, Juettler E, et al. ; Clinical Trial Net of the German Competence Network Stroke . Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol. 2010;68(4):435-445. [DOI] [PubMed] [Google Scholar]
- 16.Fransen PSS, Beumer D, Berkhemer OA, et al. ; MR CLEAN Investigators . MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. [DOI] [PubMed] [Google Scholar]
- 18.von Kummer R, Broderick JP, Campbell BCV, et al. . The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. [DOI] [PubMed] [Google Scholar]
- 19.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group: Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670-1674. [DOI] [PubMed] [Google Scholar]
- 20.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279-2284. [DOI] [PubMed] [Google Scholar]
- 21.Battey TWK, Karki M, Singhal AB, et al. . Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45(12):3643-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo AJ, Sheth KN, Kimberly WT, et al. . Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(6):742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth KN, Elm JJ, Molyneaux BJ, et al. . Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15(11):1160-1169. [DOI] [PubMed] [Google Scholar]
- 24.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58(1):593-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 27.Horsch AD, Dankbaar JW, Stemerdink TA, et al. ; DUST investigators . Imaging findings associated with space-occupying edema in patients with large middle cerebral artery infarcts. AJNR Am J Neuroradiol. 2016;37(5):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Jin ST, Kim YW, Kim SR, Park IS, Jo KW. Predictors of malignant brain edema in middle cerebral artery infarction observed on CT angiography. J Clin Neurosci. 2015;22(3):554-560. [DOI] [PubMed] [Google Scholar]
- 29.Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS One. 2017;12(2):e0171425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimoyama T, Kimura K, Uemura J, et al. . The DASH score: a simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci. 2014;338(1-2):102-106. [DOI] [PubMed] [Google Scholar]
- 31.Volny O, Cimflova P, Mikulik R. Ipsilateral sinus hypoplasia and poor leptomeningeal collaterals as midline shift predictors. J Stroke Cerebrovasc Dis. 2016;25(7):1792-1796. [DOI] [PubMed] [Google Scholar]
- 32.Kucinski T, Koch C, Grzyska U, Freitag HJ, Krömer H, Zeumer H. The predictive value of early CT and angiography for fatal hemispheric swelling in acute stroke. AJNR Am J Neuroradiol. 1998;19(5):839-846. [PMC free article] [PubMed] [Google Scholar]
- 33.Jüttler E, Unterberg A, Woitzik J, et al. ; DESTINY II Investigators . Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091-1100. [DOI] [PubMed] [Google Scholar]
- 34.Vahedi K, Hofmeijer J, Juettler E, et al. ; DECIMAL, DESTINY, and HAMLET investigators . Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222. [DOI] [PubMed] [Google Scholar]
- 35.Neuhaus AA, Couch Y, Hadley G, Buchan AM. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. 2017;140(8):2079-2092. [DOI] [PubMed] [Google Scholar]
- 36.Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW. Mass effect and death from severe acute stroke. Neurology. 1997;49(4):1090-1095. [DOI] [PubMed] [Google Scholar]
- 37.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314(15):953-958. [DOI] [PubMed] [Google Scholar]
- 38.Bucker A, Boers AM, Bot JCJ, et al. ; MR CLEAN Trial Investigators (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) . Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-hour versus 1-week imaging. Stroke. 2017;48(5):1233-1240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Method of Midline Shift Measurement.
eFigure 2. Steps of Mediation Analysis.
eFigure 3. Midline Shift Values on 24 Hour CT Scan (Early FU) According to (A) Reperfusion Status, (B) Recanalization Status, and (C) Treatment Allocation.
eTable. Common Odds Ratios (cOR) Expressing the Association between Continuous MLS and a Shift in the Direction of Worse Outcome on the mRS 90 Days.