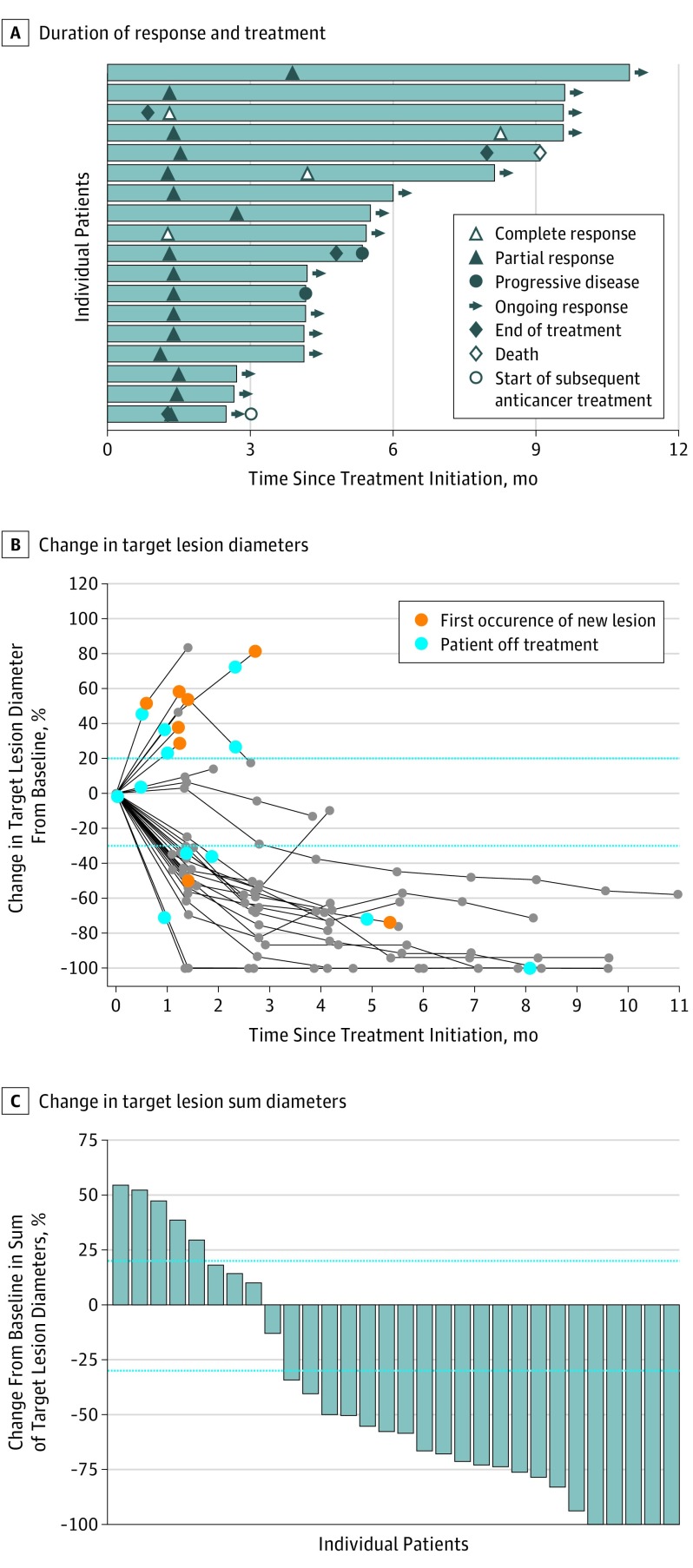
Figure 2. Clinical Activity of Avelumab.
A, Time to and duration of response and duration of treatment were measured in 18 patients with a confirmed response within the analysis set of patients with at least 3 months of follow-up. B and C, Change in target lesion diameters and percentage of change in target lesion sum diameters from baseline in individual patients were measured in 30 patients with a follow-up tumor assessment available. Parallel broken lines represent Response Evaluation Criteria in Solid Tumors values for progressive disease (≥20% increase in the sum of diameters of target lesions) and partial response (≥30% decrease in the sum of diameters of target lesions).