Figure 3. The biology of CD38 and topological paradox.
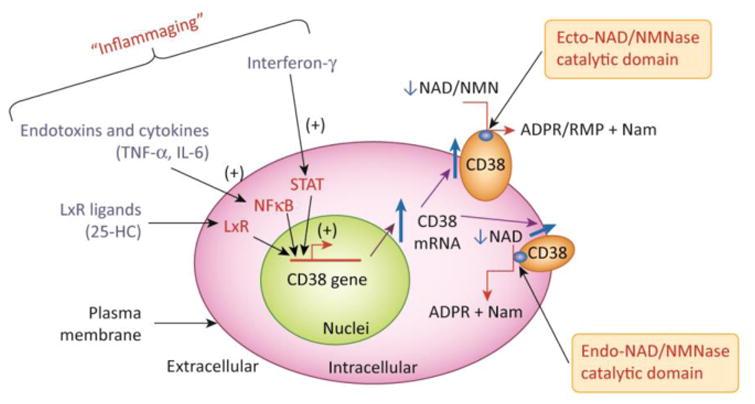
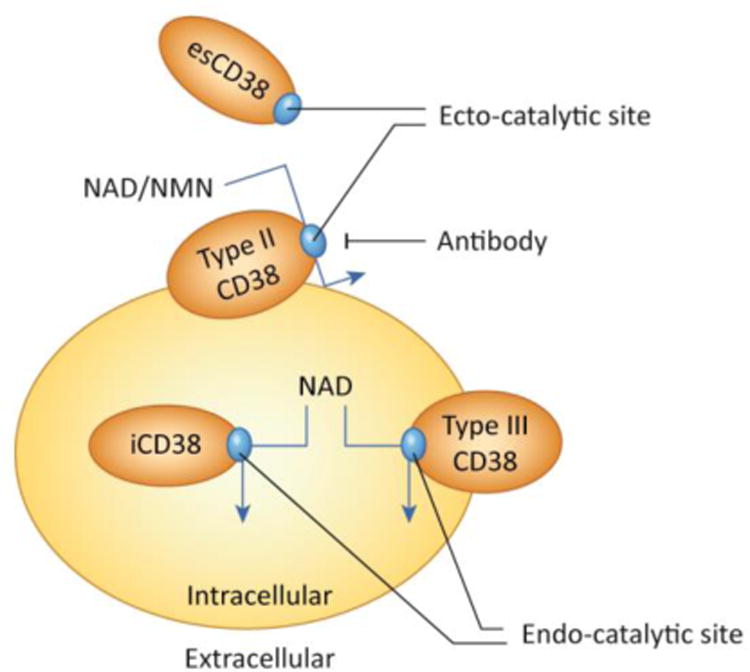
Figure 3A demonstrates the regulation of the CD38 gene expression by inflammatory agents and the link between “inflammaging”, increased CD38 expression, and cellular NAD decline. CD38 expression is activated by LPS, cytokines, interferon, and L×R ligands such as 25-hydroxycholesterolcholesterol (25-HC). In particular, cytokines, interferon and endotoxins have been proposed to play a role on the sterile inflammatory process observed during aging. In B the CD38 topologic paradox dictates that although most NAD is intracellular only a minority of CD38 has its catalytic site facing the inside of the cells. Thus, the most prevalent form of CD38 (type II), that has its catalytic site facing the outside degrades extracellular NAD and NAD precursors, such as NMN, limiting its availability to NAD synthesis in intracellular pools. A secretory soluble form of CD38 with extracellular NADase activity has also been described (esCD38). On the other hand, the type III transmembrane form of CD38 has its catalytic site facing the inside, hence having accessibility to the larger intracellular NAD pools. Minor expression of CD38 has also been described in intracellular membranes such as the nuclear membrane and mitochondrial membrane. The expression of intracellular CD38 may be limited to prevent cellular NAD degradation and cellular metabolic collapse.
