Abstract
Background and Aims:
Opioids are associated with postoperative nausea, vomiting, drowsiness, and increased analgesic requirement. A nonopioid anesthesia technique may reduce morbidity, enable day care surgery, and possibly decrease tumor recurrence. We compared opioid-free, nerve block-based anesthesia with opioid-based general anesthesia for breast cancer surgery in a prospective cohort study.
Material and Methods:
Twenty four adult American Society of Anesthesiologists grade I–III patients posted for modified radical mastectomy (MRM) with axillary dissection were induced with propofol and maintained on isoflurane (0.8–1.0 minimum alveolar concentration) through i-gel on spontaneous ventilation and administered ultrasound-guided PECS 1 and 2 blocks (0.1% lignocaine + 0.25% bupivacaine + 1 mcg/kg dexmedetomidine, 30 ml). Postoperative nausea, pain scores, nonopioid analgesic requirement over 24 h, stay in the recovery room, and satisfaction of surgeon and patient were studied. Twenty-four patients who underwent MRM and axillary dissection without a nerve block under routine opioid anesthesia with controlled ventilation were the controls.
Results:
MRM and axillary dissection under the nonopioid technique was adequate in all patients. Time in the recovery room, postoperative nausea, analgesic requirement, and visual analog scale scores were all significantly less in the nonopioid group. Surgeon and patient were satisfied with good patient quality of life on day 7.
Conclusion:
Nonopioid nerve block technique is adequate and safe for MRM with axillary clearance. Compared to conventional technique, it offers lesser morbidity and may allow for earlier discharge. Larger studies are needed to assess the long-term impact on chronic pain and tumor recurrence by nonopioid techniques.
Keywords: Analgesics, nerve block, mastectomy, non-narcotic, opioid, opioid analgesics, opioid-free anesthesia, PECS, radical
Introduction
Breast cancer is a common oncologic diagnosis in women of a productive age group. Modified radical mastectomy (MRM) with axillary clearance of lymph nodes is traditionally performed under general anesthesia with intra and postoperative opioid-based analgesia. It is associated with more than 30% incidence of postoperative nausea vomiting (PONV) and 40% acute postoperative and chronic debilitating pain.[1] Opioid-based anesthesia is associated with increased nausea and vomiting, respiratory depression, prolonged sedation, urine retention, ileus, increased postoperative pain (hyperalgesia), tolerance, and chronic pain. The possibility of higher risk of metastasis has also been reported.[2,3,4,5,6,7]
In recent times, there has been a move toward opioid-free anesthesia (OFA) to achieve the goals of hypnosis with amnesia and sympathetic stability without the adverse effects of opioids.[8,9] Various methods, such as regional blocks, and drugs, such as lignocaine, dexmedetomidine, ketamine, etc., can be employed to preclude the use of opioids.[10]
Studies have shown that when used in conjunction with opioid-based general anesthesia, nerve blocks can reduce postoperative pain and opioid requirement.[11,12,13] Single-injection paravertebral block (PVB) has been shown to be an alternative to general anesthesia for breast surgeries.[14] Recent studies have found greater opioid sparing and analgesic benefits of the PECS block (a combination of PECS 1 and 2 blocks) over the PVB.[15,16,17]
With this background in mind, we hypothesized that we may be able to perform breast surgery without using any opioids at all, thereby reducing morbidity. The purpose of our study was to find out if an opioid-free PECS block-based anesthesia technique is feasible and even beneficial compared to the opioid-based general anesthesia regimen that is routinely undertaken at our center with respect to the primary outcome measures of postoperative nausea, pain relief, and analgesic requirement and secondary outcome measures of length of stay in postanesthesia care unit (PACU) and hospital and satisfaction of patients and surgeons.
Material and Methods
This study was conducted as a part of a larger trial planned to assess two modalities of analgesia for breast cancer study (registered under CTRI/2017/02/007897). It involved adult patients of American Society of Anesthesiologists I–III category with breast cancer posted for MRM with axillary dissection. The setting was the in-patient unit and the operative suite of a 450-bedded tertiary care university (public) hospital. Following written informed consent, patients were screened for and recruited to the study.
After recruitment, the patients were administered one of two types of anesthesia in the operation suite (the days and procedures being arbitrary). One anesthetist routinely administered nonopioid anesthesia with PECS block, while two others administered opioid-based general anesthesia.
Patients were followed up for 24 h after surgery for PONV, pain scores, analgesic requirement, length of stay in recovery room, and readiness for discharge on day of surgery. A follow-up call was made at 7 days after surgery to enquire about the patients’ pain and quality of life (QOL).
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the institution.
Patient selection and anesthesia technique
Patients with a history of postoperative nausea, chronic pain, prior allergy to local anesthetics (LA) or coagulopathies, or those not consenting were excluded from the study, all others being included.
After application of standard monitoring, including ECG, noninvasive blood pressure, and pulse oximetry, all patients were administered intravenous midazolam 1–2 mg and ondansetron 4 mg.
In the patients of the nonopioid group (Gr NO) an i-gel was inserted after induction with intravenous propofol (2–3 mg/kg). Patient was maintained on spontaneous ventilation (assisted if needed with pressure support to keep EtCO2 30–40 mm Hg). Isoflurane was delivered to achieve 0.8–1.0 minimum alveolar concentration (MAC). After LA infiltration under ultrasound guidance, PECS block was administered at the level of the fourth rib in the mid-axillary line. A single-prick technique (modified from the original description of Blanco et al.) was used. Keeping the needle tip in view, 20 and 10 ml of the solution (0.3 ml/kg 0.5% bupivacaine, 0.3 ml/kg 2% lignocaine with adrenaline, and 1 mcg/kg dexmedetomidine – not exceeding toxic dosage of either LA agent) was administered:first, at the level of fourth rib, below the serratus anterior and then by withdrawing the needle to lie in between the pectoralis minor and major muscles, respectively. Drug spread in the correct plane was documented and incision was allowed in 10–15 min after testing for absence of response to skin pinch stimulus with forceps. If one or more of three predefined signs (20% rise in the baseline heart rate or blood pressure, purposeful movement of limbs, or facial grimacing) was noted on incision, rescue analgesia was administered – this included Inj. paracetamol 1 g, local infiltration with 5–10 ml 1% lignocaine, and deepening of the plane of anesthesia up to 1.2 MAC. Incision was attempted again in 5 min. Block inadequacy was defined as recurrence of any of the three predefined signs after the rescue. In case of block failure, the anesthetist could administer opioids as required.
In the patients who received opioid-based general anesthesia (Gr O), the patient was induced with propofol 1–2 mg/kg, Inj. morphine 0.1–0.2 mg/kg, and vecuronium 0.8–1 mg/kg. Anesthesia was maintained with isoflurane delivered at 1.0–1.5 MAC. Additional 3–6 mg bolus of morphine or any other opioid at the discretion of the anesthetist was administered if a 20% rise in heart rate (HR) or blood pressure (BP) was observed at incision and titrated to effect.
For postoperative analgesia in Gr NO infusion, paracetamol 1 g IV was given if visual analog scale (VAS) score was 4 or more (at rest or on arm movement) or on patient demand. In case the dose exceeded 4 g of paracetamol or if VAS >6, then Inj. diclofenac 75 mg IV was supplemented.
In Gr O, the advice was fourth-hourly administration of Inj. paracetamol. Inj. diclofenac could be administered on patient demand or if VAS was >4.
Assessment of outcomes
Postoperative nausea and vomiting
PONV was defined as any nausea, retching, or vomiting occurring during the first 24 h after surgery. In the PACU and the ward, patients were asked to report nausea “which makes you uncomfortable” or an event of retching or vomiting in a yes/no format at 4-h intervals. Data were entered as PONV present/absent per patient.
Pain
VAS pain scores were recorded on a 10 cm scale half-hourly for the first hour, hourly for next 2 h, and second-hourly thereafter for 24 h. Data were entered as the VAS scores and the total number of times that analgesic was administered.
Satisfaction scores
Surgeon and patient satisfaction scores were obtained at the end of surgery and at 24 h, respectively, on a Likert scale of 1–5, with 1 being most dissatisfied and 5 being very satisfied. Overt recall of intraoperative events was also enquired of the patient.
Quality of life
A follow-up call was given at 7 days postoperatively, to assess the QOL with a validated version of the EuroQOL-5D questionnaire.[18]
Others
Observers who were blinded to the study groups recorded the intraoperative hemodynamics (noted from the anesthesia charts), volume of breast tissue excised, and length of stay in PACU.
Bias and sample size
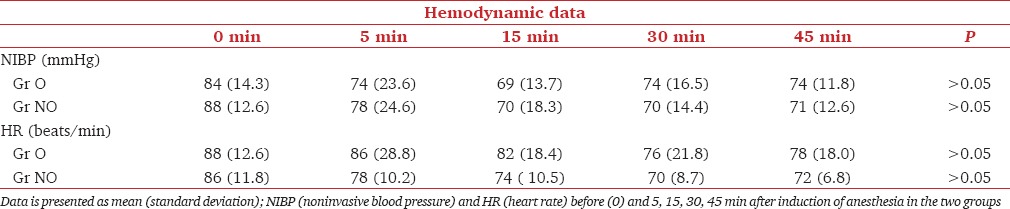
As the patients in the two subgroups of the cohort were similar with respect to age, sex, duration of anesthesia and surgery, and area in which the tumor was located Tables 1 and 2], propensity matching was not deemed necessary. Nurses in the recovery room and the general ward who assessed the nausea and pain were blinded to the treatment groups. As the block had been administered after induction and was covered under the dressings, chance of bias was reduced. Confounding was reduced by restriction-excluding patients with high risk of postoperative nausea or chronic pain (previous history of PONV, opioid medications, etc.). Nonroutine rotation of anesthetists helped in reducing patient selection bias. The sample size was estimated to have a two-sided significance level of 95%, power of 80%, and to detect a risk difference in PONV of 30% between the two groups.
Table 1.
Comparison of patient and surgery characteristics among the two groups
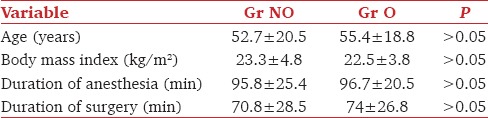
Table 2.
Intraoperative hemodynamic parameters
Statistics
Descriptive parameters were represented as means (SD) or median and range (if in skewed distribution). Continuous variables were compared using unpaired t-test (or Mann–Whitney U test) and categorical variables by Chi-square test. Statistical significance was set at P < 0.05. Missing data were handled by the multiple imputation method.
Quantitative data were categorized to allow calculation of relative risk (RR) as follows: PONV – Present/Absent; Early discharge – Was the patient considered fit for discharge within 24 h of surgery? – Yes/No; Analgesia – Did the patient require one or fewer doses of analgesia in the first 24 h after surgery? – Yes/No.
Results
Of 48 patients included in the study, 24 underwent surgery under nonopioid anesthesia and PECS block. Twenty-four underwent surgery under general anesthesia with opioid analgesia. The patient, surgery characteristics, and intraoperative hemodynamics were similar in both the groups [Tables 1 and 2].
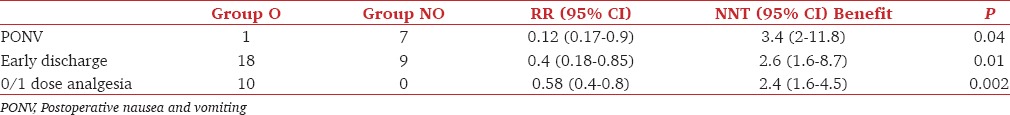
The incidence of postoperative analgesia requirement, PONV, and early discharge were compared by Chi-square test and were significantly less in Gr NO than Gr O [Table 3].
Table 3.
Postoperative outcome I
VAS scores between the two groups were compared using Mann–Whitney U test as the Shapiro–Wilk test (P < 0.05) and a visual inspection of their histograms showed that the values were not normally distributed for both the groups. A Mann–Whitney test indicated that the average VAS score was greater for Gr O than the NO group. No patient in the OFA group required diclofenac injection as compared to six patients in the O group. An independent samples t-test was used to compare the (normally distributed) time of stay in the PACU. It was shorter in Group NO than in Group O [Table 4].
Table 4.
Postoperative outcome II
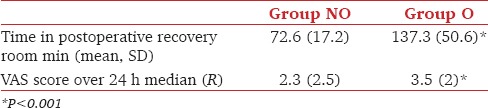
Satisfaction scores of the patients were significantly better in the NO group (P < 0.05); the first demand for analgesia was at a median of 350 min (0–1560 min) after the administration of the block. The surgeons were more satisfied with the intraoperative conditions provided in Gr O (P > 0.05).
There were no complications attributed to the PECS block. There was no incidence of bradycardia or hypotension requiring treatment in the PECS group. The EuroQOL 5D scores between the two groups were better for the NO group at 7 days of surgery (not reaching level of significance).
Discussion
We studied a cohort of patients undergoing MRM with axillary dissection for breast cancer and compared breast surgery done under PECS block without opioids with surgery done under general anesthesia with opioids. OFA under PECS block provided better analgesia (less VAS score and decreased requirement of supplemental analgesics) and reduced the PONV and duration of stay in PACU.
Previous studies involving anesthesia for breast cancer surgery aimed at identifying the best nerve block (route, dosage, site, technique, etc.), which could decrease the perioperative opioid requirement and provide better analgesia.[11,12,13,14,15,16,17] Although most studies found significant decrease in postoperative opioid requirement, none have tried to avoid opioids altogether. OFA techniques are currently gaining acceptance over the world, especially in areas of bariatric surgery and oncosurgery.[19,20,21] We demonstrate a scope for adopting this technique for breast cancer surgery as well. The benefits include avoiding respiratory depression, central muscle rigidity, pharyngeal muscle weakness, obstructed breathing, negative inotropism, nausea, vomiting, ileus and constipation, urinary retention, tolerance and addiction, dizziness, and excessive somnolence.[10]
Our method of anesthesia was finalized after some trial cases: We observed that female patients, usually anxious, find an ultrasound-guided nerve block in the chest (or back) distressing and embarrassing when awake. PECS block was chosen over PVB or other blocks as it was quicker and easier to perform and did not require a change in patient position after i-gel insertion. Recent studies have reported better block characteristics with PECS as compared to PVB.[16,17] We chose a single-point insertion nerve block technique, which gave good block results from among the many variations available for anterior chest wall nerve blocks.[21,22,23,24]
We needed a drug mixture that could provide rapid onset of block with a long duration within acceptable levels of toxicity; a mixture of lignocaine with adrenaline and bupivacaine was chosen. We added dexmedetomidine (1 mcg/kg body weight) shown to prolong block duration in nerve blocks and provide good sympathetic blockade in OFA techniques.[25,26]
We used isoflurane (age adjusted MAC of 0.8–1.0) for maintenance of anesthesia. Our choice was determined by the following factors:First, a large volume of propofol is required to keep patients sedated in OFA techniques.[27] Second, as our patients are not paralyzed, self-regulation of MAC and depth is possible with inhalation agents as compared to intravenous agents.
We noticed a steep learning curve in mastering the technique of anesthesia: Prophylactic infiltration of LA in tumors involving the upper-medial quadrant prevented the occasional arm movement at incision and gradually decreasing the inhalation agent over the course of the surgery allowed removal of i-gel by skin closure. As the patients in Gr NO recovered in the PACU, some woke up accepting the nerve block and surgery-related paresthesia quite well, whereas others appeared to have VAS pain scores >4. Some of these latter women became very comfortable once they were purposefully woken up and explained about the surgery being over; the rest needed a dose of paracetamol, often the only dose in the next 24 h.
The patient satisfaction was better in the NO group, with near pain-free arm movements. Pusch et al.[14] reported similar findings with PVB and spontaneous breathing patients. The surgeons rated the Gr O surgical experience better; this was attributed to the occasional (2 cases, early in the series) nonpurposeful movements and muscle contractions during incision and axillary clearance, respectively, in Gr NO.
There are certain limitations of our study. No attempt was made to change established practice of surgery and anesthesia in the Gr O. It is possible that use of an i-gel and avoidance of muscle relaxants may have decreased time in the recovery room in Gr O. As there was no background nerve block providing analgesia in Gr O and our group of surgeons did not desire to change practice by instituting field infiltration with LA, it was considered unethical to not provide a background cover with analgesics to this group. Thus, medical surveillance bias cannot be ruled out, as Gr O was receiving fixed eighth-hourly dose of paracetamol as opposed to Gr NO who received analgesic based on VAS. Morphine was the opioid chosen during induction based on the present theatre protocol. Although this might affect the external validity of our study (more centers use fentanyl), we hoped to mitigate the effect by avoiding postoperative patient control analgesia with morphine in both groups. Finally, observational studies are vulnerable to methodological issues, but in “ first of kind” studies they help in strengthening hypothesis and enabling sample size calculations for future randomized trials.
Conclusion
A PECS block-based anesthesia without any opioids is safe and effective. Breast surgery with axillary clearance. It appears to reduce postoperative PONV and analgesic requirement, improves the surgical experience for the patient, and may allow earlier discharge. Further studies will be needed to assess the long-term benefits of avoiding opioids.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bokhari F, Sawatzky JA. Chronic neuropathic pain in women after breast cancer treatment. Pain Manag Nurs. 2009;10:197–205. doi: 10.1016/j.pmn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol. 2010;176:786–99. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–15. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 4.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fodale V, D'Arrigo MG, Triolo S, Mondello S, La Torre D. Anesthetic techniques and cancer recurrence after surgery. Scientific World Journal. 2014;2014 doi: 10.1155/2014/328513. Article ID 328513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil SK, Anitescu M. Opioid-free perioperative analgesia for hemicolectomy in a patient with opioid-induced delirium: A case report and review of the analgesic efficacy of the alpha-2 agonist agents. Pain Pract. 2012;12:656–62. doi: 10.1111/j.1533-2500.2012.00543.x. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 8.Mulier JP. Opioid free anaesthesia (OFA) a Paradigma shift? 2015. [Last accessed on 2017 Jun 11]. Retrieved from http://www.researchgate.net/publication/278307444 .
- 9.Mulier JP, Wouters R, and Dekock M. Pourquoi et comment éviter les opioïdes en anesthésie ambulatoire? Non-opioid surgical anaesthesia. Presented at JEPU Conference, Paris. 2014 [Google Scholar]
- 10.Sultana A. [Last accessed on 2017 Jan 19];Opioid free anesthesia and analgesia in the bariatric patient. Available at: http://ispcop.net/images/ispcop/publications/education/Sultana_OFA.pdf . [Google Scholar]
- 11.Lynch EP, Welch KJ, Carabuena JM, Eberlein TJ. Thoracic epidural anesthesia improves outcome after breast surgery. Ann Surg. 1995;222:663–9. doi: 10.1097/00000658-199511000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greengrass R, O'Brien F, Lyerly K, Hardman D, Gleason D, D'Ercole, et al. Paravertebral block for breast cancer surgery. Can J Anaesth. 1996;43:858–61. doi: 10.1007/BF03013039. [DOI] [PubMed] [Google Scholar]
- 13.Weltz CR, Greengrass RA, Lyerly HK. Ambulatory surgical management of breast carcinoma using paravertebral block. Ann Surg. 1995;222:19–26. doi: 10.1097/00000658-199507000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single-injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999;43:770–4. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 15.Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: A randomized clinical trial. Reg Anesth Pain Med. 2015;40:68–74. doi: 10.1097/AAP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 16.Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: A randomized controlled trial. Br J Anaesth. 2016;117:382–6. doi: 10.1093/bja/aew223. [DOI] [PubMed] [Google Scholar]
- 17.Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egyptian J Anaesth. 2014;30:129–35. [Google Scholar]
- 18.Tripathy S, Hansda U, Seth N, Rath S, Rao PB, Mishra TS, et al. Validation of the EuroQol five-dimensions - Three-level quality of life instrument in a classical Indian language (Odia) and its use to assess quality of life and health status of cancer patients in Eastern India. Indian J Palliat Care. 2015;21:282–8. doi: 10.4103/0973-1075.164896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balandin VV, Gorobec ES. Opioid-free anesthesia, analgesia and sedation in surgery of head and neck tumor. Anesteziol Reanimatol. 2015;60:39–42. [PubMed] [Google Scholar]
- 20.Mansour MA, Mahmoud AA, Geddawy M. Nonopioid versus opioid based general anesthesia technique for bariatric surgery: A randomized double-blind study. Saudi J Anaesth. 2013;7:387–91. doi: 10.4103/1658-354X.121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco R, Fajardo M, Parras T. Ultrasound description of Pecs II (modified Pecs I): A novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470–5. doi: 10.1016/j.redar.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty A, Khemka R, Datta T, Mitra S. COMBIPECS, the single-injection technique of pectoral nerve blocks 1 and 2: A case series. J Clin Anesth. 2016;35:365–8. doi: 10.1016/j.jclinane.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian MP. Pecs II or serratus plane block? Anaesthesia. 2014;69:1173. doi: 10.1111/anae.12822. [DOI] [PubMed] [Google Scholar]
- 24.Del Buono R, Costa F, Agrò FE. Parasternal, pecto-intercostal, Pecs, and transverse thoracic muscle plane blocks: A rose by any other name would smell as sweet. Reg Anesth Pain Med. 2016;41:791–2. doi: 10.1097/AAP.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 25.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: A systematic review and meta-analysis. Br J Anaesth. 2013;110:915–25. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 26.Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity. Anesthesiology. 2012;116:1312–22. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 27.Castro A. [Last accessed on 2017 May 02];Opioid free anaesthesia [PDF Document] Retrieved from http://anaesthetics.ukzn.ac.za/Libraries/ICU_1/FMM_No5_04-03-2016_OFA_A_de_Castro.sflb.ashx . [Google Scholar]


