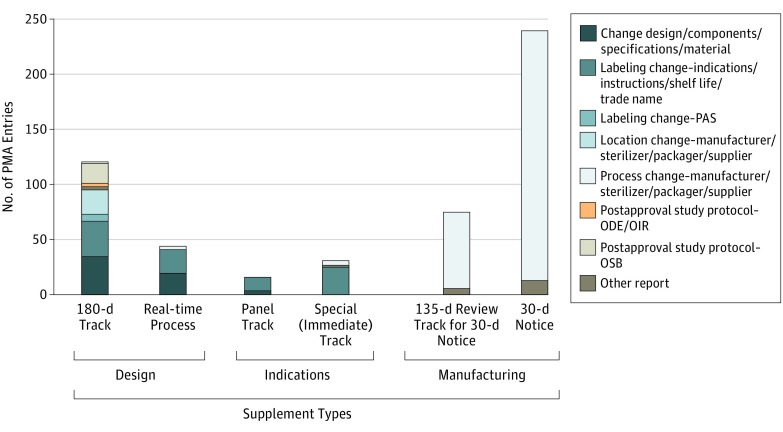
Figure 3. Types of Changes in Dermatologic Devices by Supplement Track.
Unclassified supplements are not included in this graph. A sizable proportion of the 180-day track supplements are used by manufacturing changes, rather than design and labeling changes. A significant level of the real-time process applications was approved for labeling and manufacturing changes, rather than its intended use for minor design modifications. Panel track and special (immediate track) supplements were used for labeling changes, with few exceptions. Last, 30-day and 135-day tracks were appropriately used for manufacturing changes. ODE indicates Office of Device Evaluation; OIR, Office of In Vitro Diagnostics and Radiological Health; OSB, Office of Surveillance and Biometrics; PAS, postapproval study; and PMA, premarket approval.