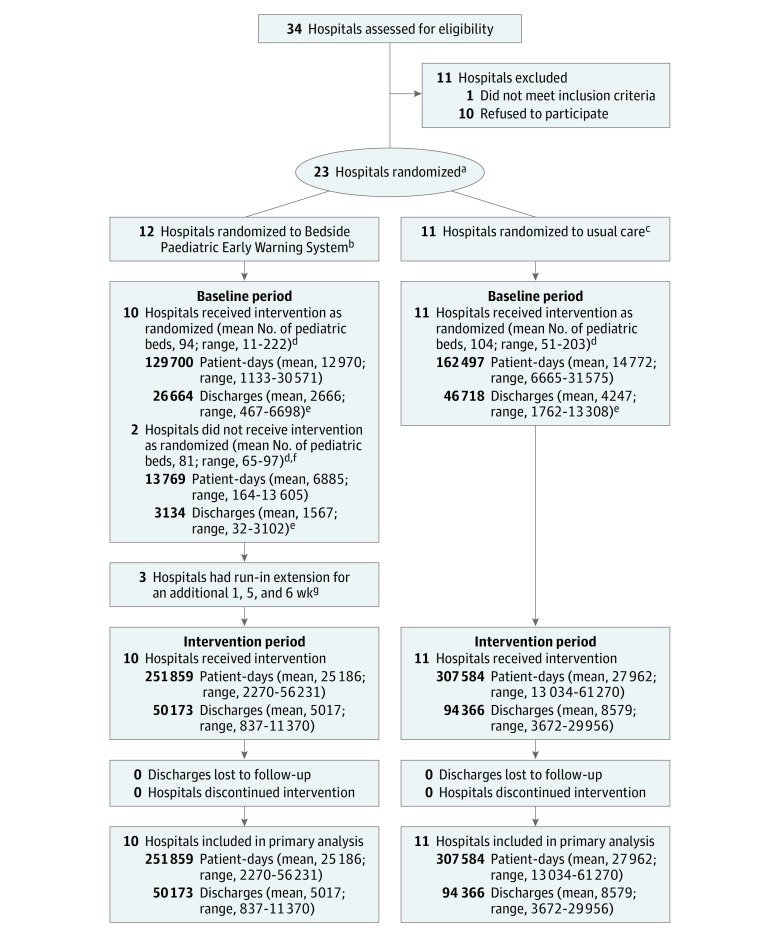
Figure 1. Hospital Site and Patient Flow Through Study.
aEnrollment began in 2011 for 8 hospitals, in 2012 for 10 hospitals, and in 2013 for 5 hospitals. Disclosure of randomization to the hospital occurred during the second week of the 26-week baseline period.
bHospitals randomized to the BedsidePEWS intervention collected data during the 26-week baseline period as they prepared for implementation of the intervention. During the 5-week run-in phase, adherence to vital sign documentation was assessed and reported to the study executive steering committee. Implementing hospitals required a minimum of 80% adherence to documentation standards and the majority vote of the executive steering committee to move into the intervention period.
cHospitals randomized to usual care collected data during the 26-week baseline period, did not collect data during the 5-week run-in phase, and then resumed data collection for the 52-week intervention period.
dDid not include beds in the intensive care unit.
eDischarges were all eligible patients discharged from the hospital and included the patients who died. Patients who were in the hospital at the end of the study period were regarded as discharged.
fUnable to adhere to implementation timelines specified by the study and were excluded before implementation. These hospitals were regional pediatric centers. One hospital had a rapid response team and no extracorporeal membrane oxygenation and the other did not have a rapid response team but had extracorporeal membrane oxygenation. The numbers of patient-days and patient discharges reported reflect the amount of data received from each hospital before they withdrew from the study.
gThe 5-week run-in phase included weekly assessment beginning during the second week of implementation.