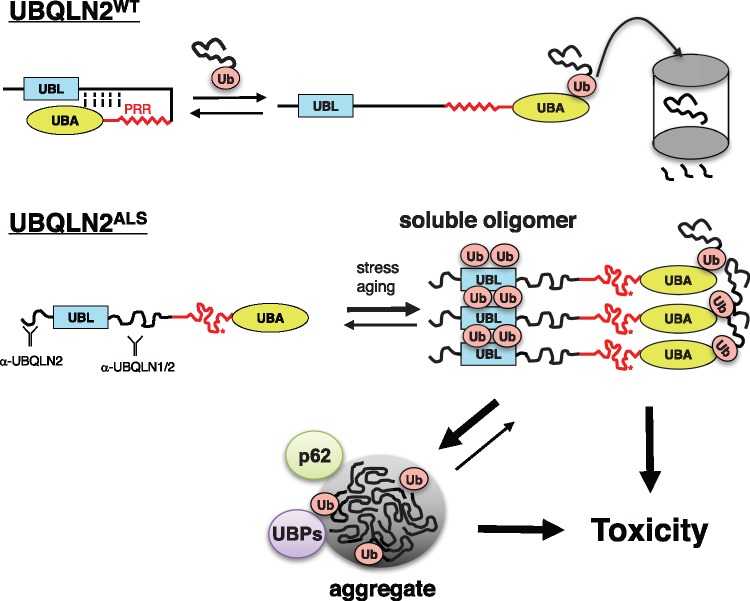
Figure 8.
Model for coupled misfolding, Ub-binding and ubiquitylation of UBQLN2ALS mutants. UBQLN2WT cycles between folded and open, Ub bound conformations during its activities as a Ub chaperone. PRR mutations result in misfolding of UBQLN2, leading to exposure of epitopes, increased oligomerization, and increased Ub engagement. UBQLN2 oligomers may become insoluble or engage additional Ub moieties via enhanced Ub-binding potential. Both soluble and aggregated UBQLN2 proteins are proposed to instigate toxicity. UBP, Ub-binding protein.