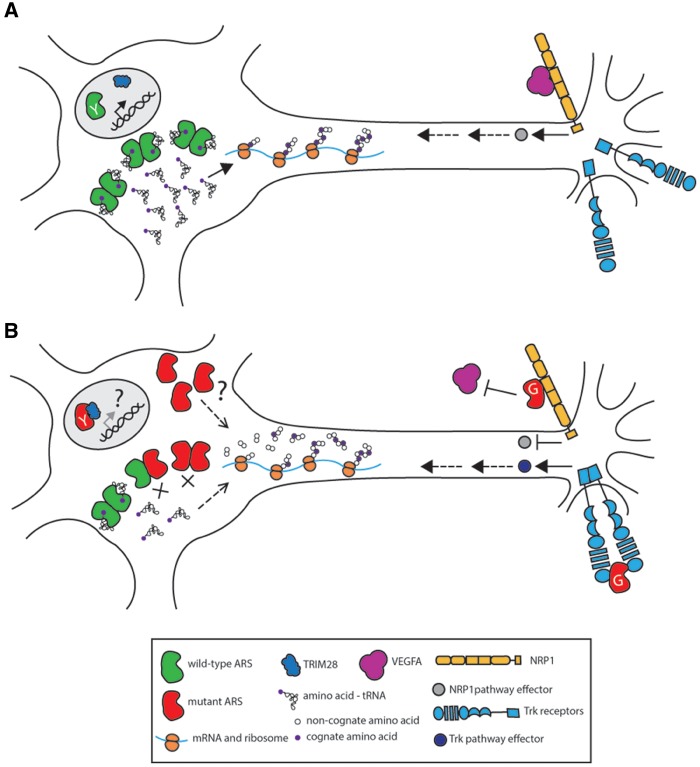
Figure 2.
Potential mechanisms of ARS-related dominant axonal neuropathy. Neurons are illustrated with the cell body on the left and the axon extending to the right. A wild-type neuron (A) has functional ARS activity (green dimers) facilitating protein translation. There is appropriate NRP1 (orange transmembrane protein) and Trk signaling (blue transmembrane protein). YARS translocates to the nucleus upon oxidative stress and binds TRIM28 (blue), potentially changing the regulation of DNA damage response genes. Proposed mechanisms of ARS-mediated peripheral neuropathy are represented in (B); see text for details. Neuronal function may be compromised by impaired protein translation due to an unknown function of mutant ARS (red subunits) and/or a depletion in available charged tRNA from a significant reduction of aminoacylation activity. For peripheral neuropathy related to GARS mutations, mutant GARS may interfere with NRP1 signaling by preventing VEGFA (magenta) from binding to NRP1. In developing sensory neurons, mutant GARS may also act as a ligand for Trk receptors, aberrantly activating Trk signaling. For peripheral neuropathy related to YARS mutations, increased mutant YARS binding to TRIM28 (blue) may change the expression of DNA damage response genes.