Abstract
Fanconi–Bickel syndrome, caused by mutations in SLC2A2 encoding the glucose transporter 2 (GLUT2), is characterized by generalized proximal renal tubular dysfunction manifesting in late infancy. We describe phenotypic heterogeneity of Fanconi–Bickel syndrome in three siblings, including early and atypical presentation with transient neonatal diabetes mellitus in one. The second-born of a non-consanguineous couple, evaluated for polyuria and growth retardation, had rickets, hepatomegaly and proximal tubular dysfunction from 4 to 6 months of age. A male sibling, who expired at 4 months, also had hepatomegaly and growth retardation. The third sibling had polyuria, glucosuria and mild proteinuria on day 3 of life. Hyperglycemia was detected 2 weeks later, which required therapy with insulin for 3 months. Mild metabolic acidosis was present at 2 weeks; hypercalciuria, phosphaturia and aminoaciduria were seen at 6 months. Sanger sequencing showed a homozygous missense mutation in SLC2A2 (exon 7, c.952G > A), causing glycine to arginine substitution; both parents were heterozygous carriers. Patients with SLC2A2 mutations may present either with isolated neonatal diabetes or with hepatomegaly and the renal Fanconi syndrome. Fanconi–Bickel syndrome shows phenotypic heterogeneity and may manifest early with subtle or atypical features, mandating a high index of suspicion.
Keywords: Fanconi–Bickel syndrome, Neonatal diabetes mellitus, Sodium–glucose transporter 2, Renal tubular acidosis, Genetic pleiotropy
Background
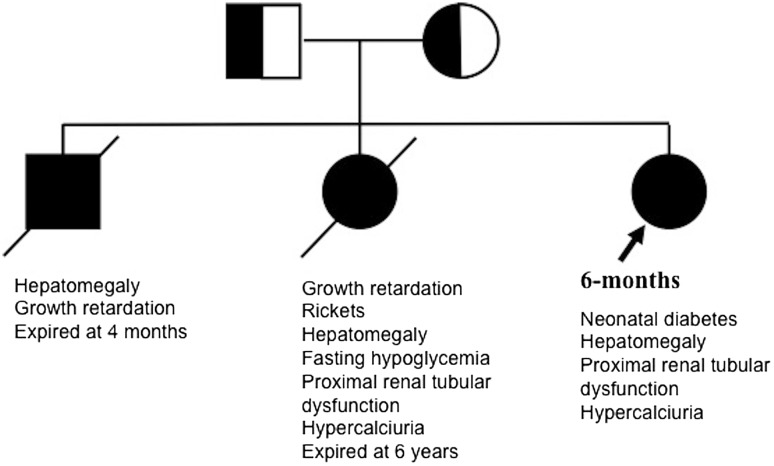
Fanconi–Bickel syndrome, a disorder of carbohydrate metabolism, is characterized by hepatomegaly, fasting hypoglycemia, postprandial hyperglycemia, generalized proximal tubular dysfunction, rickets and growth retardation. This autosomal recessively inherited disease is caused by homozygous or compound heterozygous mutations in the SLC2A2 gene, mapped on chromosome 3q26.1-26.3, encoding the facultative glucose transporter 2 (GLUT2) [1]. While multiple mutations are described, there are few mutational hotspots and no genotype–phenotype correlation [2–4]. Variation in disease severity with identical mutations has also been reported [2, 5]. Patients typically present in infancy with vomiting, growth failure and refractory rickets [3]. Presentation in neonates is uncommon, and diabetes mellitus is extremely rare [6]. We describe phenotypic heterogeneity of Fanconi–Bickel syndrome in three siblings (Fig. 1), where genetic testing in the youngest child, presenting with transient neonatal diabetes, revealed a homozygous mutation in SLC2A2.
Fig. 1.
Family of three siblings with Fanconi–Bickel syndrome. Sanger sequencing showed a homozygous c.952G > A (p.Gly318Arg) mutation in SLC2A2 in the youngest child who presented with transient neonatal diabetes mellitus. Parents were heterozygous carriers while affected elder siblings were not tested
Case report
A 6-month-old girl, born of a non-consanguineous marriage, presented with polyuria, poor growth and recurrent vomiting since 4 months of life. There was no history of seizures or jaundice; antenatal and postnatal course were unremarkable. An elder male sibling evaluated elsewhere for failure to thrive, polyuria and hepatomegaly at 3 months of age, had succumbed to an illness with fast breathing. Examination showed severe undernutrition with weight of 4.5 kg and length 57 cm (both < − 3 standard deviation scores, SDS). There was rachitic rosary and liver was enlarged 4 cm below the right costal margin (span 7 cm). Muscle tone was normal and deep tendon reflexes were elicited normally.
The arterial pH was7.38, bicarbonate 15.6 mEq/l and anion gap 8 mEq/l; the corresponding urine pH was 5.0. Following bicarbonate loading, fractional excretion of bicarbonate and the difference between urine and blood PCO2 were 20% and 28 mmHg, respectively, suggesting proximal tubular acidosis. Blood investigations showed phosphorus level of 2.1–2.3 mg/dl, potassium 2.7–3.1 mEq/l, alkaline phosphatase 3156 IU/l (normal 145–420 IU/l), and aspartate and alanine aminotransferase156 IU/l and 56 IU/l, respectively. Blood levels of calcium (9.8 mg/dl), creatinine (0.2 mg/dl), sodium (135 mEq/l), uric acid (2.0 mg/dl), lactate (0.9 mEq/l), albumin (3.7 g/dl) and bilirubin (0.2 mg/dl) were normal. Urinalysis showed glucosuria, generalized aminoaciduria and increased excretion of β2-microglobin (40000 ng/ml; normal < 300 ng/ml). Tubular maximum for phosphate reabsorption/glomerular filtration rate (TmP/GFR) was 0.5 mg/dl (normal 2.9–4.6 mg/dl) and calcium excretion was 13 mg/kg/day (normal < 4 mg/kg/day); nephrocalcinosis was not detected. Corneal cystine crystal deposition was absent, activity of galactose-1-phosphate uridyltransferase was normal and urinary succinylacetone was undetectable.
She was lost to follow-up till 4 years of age, when she presented with severe rickets and recurrent fractures, weight 6.2 kg and height 69 cm (both < − 3SDS). Liver was palpable 6 cm below costal margin with a span of 10 cm. Fasting hypoglycemia was detected with glucose ranging from 36 to 45 mg/dl on multiple occasions. A diagnosis of Fanconi–Bickel syndrome was made in presence of proximal renal tubular dysfunction, hypercalciuria, fasting hypoglycemia, rickets and hepatomegaly. Following nutritional rehabilitation, supplements of bicarbonate, phosphate and potassium were advised. Compliance and follow-up was erratic; the patient succumbed at home 2 years later during a febrile illness.
The third sibling, a girl, was born by spontaneous vaginal delivery at gestation of 40 weeks with weight of 2.3 kg. While perinatal period was uneventful, parents observed polyuria on third day of life. There was no vomiting, fever, lethargy or seizures. Urinalysis showed pH 5.0, glucosuria (3+) and trace proteinuria. Hyperglycemia (blood sugar 250–400 mg/dl) was detected 2 weeks later. Serum C-peptide level was 0.23 ng/ml (normal 0.78–5.19 ng/ml). At presentation, the 6-week-old girl weighed 4.1 kg (− 2 SDS); length was 55.5 cm (− 1 SDS) and head circumference 37 cm (− 1 SDS). Investigations showed normal blood levels of creatinine (0.2 mg/dl), sodium (136 mEq/l), potassium (3.8 mEq/l), chloride (110 mEq/l), calcium (8.7 mg/dl) and phosphorus (4.1 mg/dl); alkaline phosphatase was elevated (3733 IU/l). Blood pH was 7.32, bicarbonate 18 mEq/l and anion gap 8 mEq/l.
The patient was treated with insulin to achieve euglycemia. Considering the diagnosis of transient neonatal diabetes mellitus with Fanconi–Bickel syndrome, Sanger sequencing of SLC2A2 gene was performed. A homozygous missense mutation in exon 7 of SLC2A2 (c.952G > A) was detected in the patient, with parents as heterozygous carriers. This mutation, reported previously in Fanconi–Bickel syndrome [3], was considered damaging by in silico pathogenicity prediction software PolyPhen2 (score 0.999; http://genetics.bwh.harvard.edu/pph2) and sorting intolerant from tolerant (SIFT score 0; http://sift.jcvi.org). During follow-up, insulin requirement declined and disappeared by 3 months of age. Uncooked corn starch-based diet was introduced. Polyuria persisted (urine output 11 ml/kg/hr); the liver was palpable 3 cm below costal margin at 3 months of age. At 6 months, the child weighs 6.6 kg (− 1 to − 2 SDS) with urinary calcium excretion of 12.3 mg/kg/day, phosphaturia (TmP/GFR 1.2 mg/dl) and generalized aminoaciduria.
Discussion
We report three siblings with Fanconi–Bickel syndrome presenting with either renal Fanconi syndrome or transient neonatal diabetes mellitus. The condition was suspected clinically in one child with proximal tubular dysfunction, hepatomegaly and fasting hypoglycemia. The diagnosis was confirmed when the youngest sibling presented with neonatal diabetes, an entity with relatively fewer monogenic etiologies compared to Fanconi syndrome [7, 8]. While phenotypic heterogeneity is described for mutations in SLC2A2 encoding GLUT2, variations in phenotype within families, development of diabetes and neonatal presentation are uncommon [2, 5, 9–11].
GLUT2, a glucose transporter located on the basolateral membrane of enterocytes and proximal convoluted tubule cells, facilitates transepithelial glucose transport initiated by the apical sodium–glucose cotransporter [12]. Defects in GLUT2 cause hepatomegaly due to glycogen accumulation, and glucosuria due to impaired proximal tubular reabsorption. However, the pathophysiology of Fanconi syndrome, first described in an 8-year-old boy with microalbuminuria and mesangial expansion and similar homozygous SLC2A2 mutation (c.952G > A) as our patient [3, 13], remains unclear. Postulated mechanisms are downregulation of surface expression of transporters, impaired mitochondrial metabolism and reduced solute reclamation due to solvent drag caused by high intracellular glucose concentration [12, 13]. The missense mutation in our patient that substitutes glycine with arginine at position 318 (p.G318R), has been reported in two patients with Fanconi–Bickel syndrome [3, 13], none of whom had diabetes mellitus (Table 1).
Table 1.
Phenotypic heterogeneity in patients with Fanconi–Bickel syndrome (FBS) with homozygous p.Gly318Arg mutation in SLC2A2
| Sex; country [ref] | Birth weight, gestation | Features of FBS | Unique features | Age at onset | Status at follow-up |
|---|---|---|---|---|---|
| Girl; Italy [3, 14] | NA | Increased liver glycogen content, hepatomegaly | Cataracts | Reported at 13 years | NA |
| Boy; USA [3, 13] | NA | Growth retardation, hepatomegaly, rickets, fasting hypoglycemia, proximal RTA, hypophosphatemia, hypouricemia | Microalbuminuria, glomerular hyperfiltration and diffuse mesangial expansion | 3 years | Wheelchair bound by 8 years (severe rickets) |
| Boy; Indiaa,b (present case) | 2.4 kg 40 weeks |
Growth retardation, polyuria, hepatomegaly | None | 3 months | Expired at 4 months |
| Girl; Indiaa,b (present case) | 2.8 kg 40 weeks |
Growth retardation, rickets, hepatomegaly, fasting hypoglycemia, proximal RTA, phosphaturia, generalized aminoaciduria, glucosuria, hypercalciuria | None | 4 months | Expired at 6 years |
| Girl Indiaa (present case) | 2.3 kg 40 weeks |
Hepatomegaly, phosphaturia, hypercalciuria, aminoaciduria, proximal RTA | Transient neonatal diabetes mellites from day 3 | 2 weeks; subtle features day 3 | Presently 6 months |
NA not available; RTA renal tubular acidosis
aSiblings of a non-consanguineous marriage
bGenetic testing not done, presumed to have similar mutation as sibling; both parents heterozygous carriers
Transient or permanent neonatal diabetes is an unusual feature of Fanconi–Bickel syndrome, reported in seven of 150 patients with homozygous SLC2A2 mutations [4, 6, 9, 15, 16]. Neonatal diabetes is predominantly a monogenic disorder with causative mutation identified in one of 22 genes in 80% patients [7, 17]. While mutations in the potassium channel subunit genes, ABCC8 and KCNJ11account for 40% cases [7], SLC2A2 mutations are seen in only 5% patients [9]. The pathophysiology of diabetes in Fanconi–Bickel syndrome may relate to impaired glucose uptake for glucose-stimulated insulin secretion by the pancreatic beta cells. However, while SLC2A2 knockout mice die with neonatal diabetes [18], GLUT1 and GLUT3 receptors compensate for absent GLUT2 in human pancreatic beta cells [12]. It is proposed that insulin secretion in neonates is transiently controlled by GLUT2 or that undiagnosed defects in GLUT1 and GLUT3 contribute to diabetes [12]. The transient nature of hyperglycemia may be related to the low pancreatic beta cell mass in neonates that increases through infancy [12]. Most infants with the Fanconi–Bickel syndrome and diabetes have intrauterine growth retardation [9], underscoring the role of insulin in fetal growth [17]. Three such patients presented with low C-peptide levels [4, 9], resembling our patient and suggesting insulin deficiency.
Similar to the present report, the onset of hyperglycemia in patients with diabetes due to GLUT2 defects has been reported to occur as early at day 1 to 42 of life, lasting variably up to 18 months [4, 6, 9, 15, 16]. While development of Fanconi–Bickel syndrome early in infancy is described in four of these patients [4, 6, 9, 15, 16], the clinical features may be delayed by several months, reported between 45 days and 48 months [6, 9]. In contrast, the youngest sibling in the present report showed proteinuria by day 3 of life; hyperchloremic metabolic acidosis and elevated alkaline phosphatase was detected by 6 weeks. Neonatal presentation is uncommon in Fanconi–Bickel syndrome, although newborn screening may show hypergalactosemia [10, 11].
Table 1 shows phenotypic heterogeneity of the p.G318R mutation in five patients with Fanconi–Bickel syndrome. None of the other patients were reported to manifest with neonatal diabetes. The reason for variable clinical phenotype among siblings of the same family, with presumed identical mutations, as noted in the present and two other families with different mutations is unclear [9]. While a limitation of this report is that mutation analyses of two elder siblings of our patient could not be performed, they possibly had identical homozygous mutation and were not diagnosed with neonatal diabetes but presented with features of Fanconi–Bickel syndrome.
Careful evaluation of families with the Fanconi–Bickel syndrome allows an understanding the course and spectrum of the illness. Mutations in SLC2A2 must be considered in patients with familial proximal renal tubular acidosis and neonatal onset of acidosis or glucose intolerance, with or without hepatomegaly. Specific genetic testing may enable prenatal counseling and help predict the clinical phenotype.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the parents of the patients included in this article.
References
- 1.Santer R, Schneppenheim R, Dombrowski A, Gotze H, Steinmann B, Schaub J. Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi–Bickel syndrome. Nat Genet. 1997;17(3):324–326. doi: 10.1038/ng1197-324. [DOI] [PubMed] [Google Scholar]
- 2.Fridman E, Zeharia A, Markus-Eidlitz T, Haimi Cohen Y. Phenotypic variability in patients with Fanconi–Bickel Syndrome with identical mutations. JIMD Rep. 2015;15:95–104. doi: 10.1007/8904_2014_303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santer R, Groth S, Kinner M, Dombrowski A, Berry GT, Brodehl J, et al. The mutation spectrum of the facilitative glucose transporter gene SLC2A2 (GLUT2) in patients with Fanconi–Bickel syndrome. Hum Genet. 2002;110(1):21–29. doi: 10.1007/s00439-001-0638-6. [DOI] [PubMed] [Google Scholar]
- 4.Yoo HW, Shin YL, Seo EJ, Kim GH. Identification of a novel mutation in the GLUT2 gene in a patient with Fanconi–Bickel syndrome presenting with neonatal diabetes mellitus and galactosaemia. Eur J Pediatr. 2002;161(6):351–353. doi: 10.1007/s00431-002-0931-y. [DOI] [PubMed] [Google Scholar]
- 5.Dweikat IM, Alawneh IS, Bahar SF, Sultan MI. Fanconi–Bickel syndrome in two Palestinian children: marked phenotypic variability with identical mutation. BMC Res Notes. 2016;9:387. doi: 10.1186/s13104-016-2184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setoodeh A, Rabbani A. Transient neonatal diabetes as a presentation of Fanconi–Bickel syndrome. Acta Med Iran. 2012;50(12):836–838. [PubMed] [Google Scholar]
- 7.De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. The Lancet. 2015;386(9997):957–963. doi: 10.1016/S0140-6736(15)60098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor RN, Greeley SA, Bell GI, Philipson LH. Genetics and pathophysiology of neonatal diabetes mellitus. J Diabetes Investig. 2011;2(3):158–169. doi: 10.1111/j.2040-1124.2011.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansbury FH, Flanagan SE, Houghton JA, Shuixian Shen FL, Al-Senani AM, Habeb AM, et al. SLC2A2 mutations can cause neonatal diabetes, suggesting GLUT2 may have a role in human insulin secretion. Diabetologia. 2012;55(9):2381–2385. doi: 10.1007/s00125-012-2595-0. [DOI] [PubMed] [Google Scholar]
- 10.Riva S, Ghisalberti C, Parini R, Furlan F, Bettinelli A, Somaschini M. The Fanconi–Bickel syndrome: a case of neonatal onset. J Perinatol. 2004;24(5):322–323. doi: 10.1038/sj.jp.7211092. [DOI] [PubMed] [Google Scholar]
- 11.Peduto A, Spada M, Alluto A, La Dolcetta M, Ponzone A, Santer R. A novel mutation in the GLUT2 gene in a patient with Fanconi–Bickel syndrome detected by neonatal screening for galactosaemia. J Inherit Metab Dis. 2004;27(2):279–280. doi: 10.1023/B:BOLI.0000028841.00833.f4. [DOI] [PubMed] [Google Scholar]
- 12.Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221–32. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- 13.Berry GT, Baker L, Kaplan FS, Witzleben CL. Diabetes-like renal glomerular disease in Fanconi–Bickel syndrome. Pediatr Nephrol. 1995;9(3):287–291. doi: 10.1007/BF02254185. [DOI] [PubMed] [Google Scholar]
- 14.Santer R, Schneppenheim R, Suter D, Schaub J, Steinmann B. Fanconi–Bickel syndrome–the original patient and his natural history, historical steps leading to the primary defect, and a review of the literature. Eur J Pediatr. 1998;157(10):783–797. doi: 10.1007/s004310050937. [DOI] [PubMed] [Google Scholar]
- 15.Habeb AM, Al-Magamsi MS, Eid IM, Ali MI, Hattersley AT, Hussain K, et al. Incidence, genetics, and clinical phenotype of permanent neonatal diabetes mellitus in northwest Saudi Arabia. Pediatr Diabetes. 2012;13(6):499–505. doi: 10.1111/j.1399-5448.2011.00828.x. [DOI] [PubMed] [Google Scholar]
- 16.Jahnavi S, Poovazhagi V, Mohan V, Bodhini D, Raghupathy P, Amutha A, et al. Clinical and molecular characterization of neonatal diabetes and monogenic syndromic diabetes in Asian Indian children. Clin Genet. 2013;83(5):439–445. doi: 10.1111/j.1399-0004.2012.01939.x. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev. 2008;29(3):265–291. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17(3):327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]