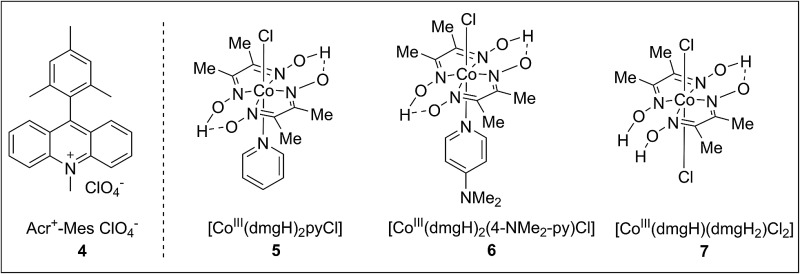
Table 1. Optimization studies a .

| |||
| Entry | Catalyst | Solvent | Yield b (%) |
| 1 | Co(dmgH)pyCl | CH3CN | 17 (20 : 1) |
| 2 | Co(dmgH)pyCl | THF | 14 (7 : 1) |
| 3 | Co(dmgH)pyCl | DCE | 44 (7 : 1) |
| 4 | Co(dmgH)2(4-NMe2py)Cl | DCE | 70 (12 : 1) |
| 5 | Co(dmgH)(dmgH2)Cl2 | DCE | 14 (2 : 1) |
| 6 | None | DCE | n.d. |
| 7 c | Co(dmgH)2(4-NMe2py)Cl | DCE | n.d. |
| 8 d | Co(dmgH)2(4-NMe2py)Cl | DCE | n.d. |
| |||
aConditions: 1a (0.20 mmol), 2a (0.4 mmol), Acr+-Mes ClO4– (3 mol%) and co-catalyst (10 mol%) were added in solvent (2 mL) under N2 and irradiated by blue LEDs for 24 h.
bYields were determined by GC with naphthalene as the internal standard and are the sum of the cis and trans isomers. The ratio of the E/Z isomers are shown in parenthesis.
cReaction was performed without photosensitizer.
dReaction was carried out in the dark.
