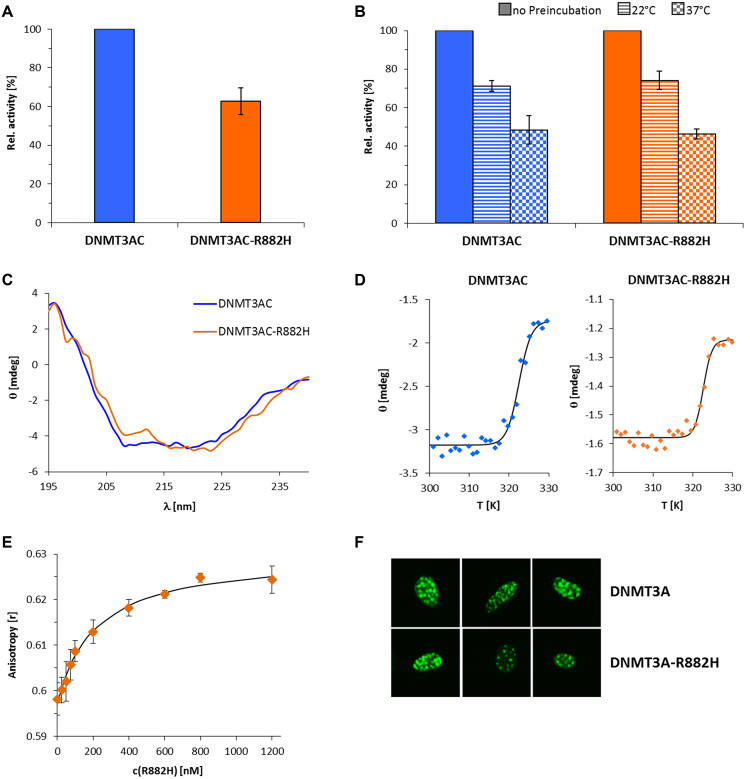
Figure 1.
Catalytic activity and characterization of the R882H mutant. (A) DNA methylation activity of DNMT3A catalytic domain and the R882H mutant using a 509mer DNA substrate. The bars represent the average of 5 experiments, error bars indicate the standard error of the mean. See also Supplementary Figure S3. (B) Protein stability of wildtype DNMT3AC and R882H analyzed by the loss of enzymatic activity during a pre-incubation of the protein for 30 min at different temperatures. Error bars indicate the SEM based on 2 independent experiments. (C) CD spectra of wildtype DNMT3AC and R882H. The similar shape of the spectra indicates comparable folding of both proteins. (D) Thermal stability of DNMT3AC and R882H analyzed by CD melting at protein concentrations of 22 μM for DNMT3AC and 8 μM for R882H. Quantitative analysis revealed identical melting temperatures of 49.3 ± 0.4°C and 49.5 ± 0.6°C for DNMT3AC and R882H. (E) DNA binding titration of the R882H mutant to a 29mer DNA substrate. The data points show the average of two experiments and the standard error. The line shows a fit of the data with a KD of 209 nM. (F) Heterochromatic localization of EYFP fused murine DNMT3A containing the R878H mutation (corresponding to R882H in human DNMT3A).