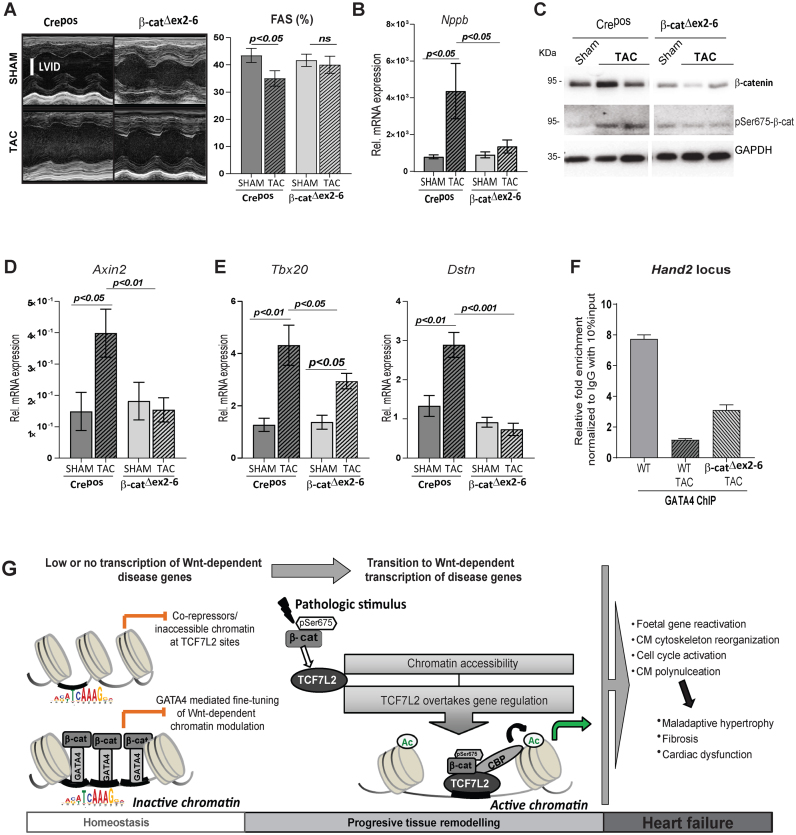
Figure 8.
β-catenin loss of function in CM rescues Wnt-dependent pathological gene regulation in vivo. (A) Representative examples of M-mode echocardiograms, and quantification of fractional shortening (FAS) by echocardiographic analysis of 3 weeks TAC-induced Crepos control and β-catΔex2–6 mice, n ≥ 7. (B) Relative transcript levels of hypertrophic marker Nppb in β-catΔex2–6 and controls Crepos; sham and TAC (n≥8/per group). (C) Immunoblot depicting total β-catenin and pSer675-β-catenin upon TAC in β-catenin loss of function (β-catΔex2–6). GAPDH was protein-loading control (n = 2/group). Relative transcript levels of (D) classical Wnt target Axin2 and (E) newly identified cardiac Wnt targets Tbx20 and Dstn, 6 weeks post-TAC in β-catΔex2–6 and controls, n≥7. Data are mean ± SEM; ANOVA, Bonferroni's multiple comparison test. Tbp was used for transcript normalization. (F) ChIP-qPCR for GATA4 binding on Hand2 enhancer in normal (WT), 6 weeks post-TAC (WT TAC) and β-catΔex2–6 TAC hearts. Relative fold enrichment was calculated to IgG control, normalized to 10% input chromatin (n = 3 hearts/ChIP). (G) Schematic representation of the findings of this study. In the healthy adult heart, β-catenin/TCF7L2-dependent loci are inactive, inaccessible or bound by transcriptional repressors, resulting in low transcription. On a subset of these loci, GATA4 binds to transcriptionally inactive β-catenin, fine-tuning Wnt-dependent transcription. This chromatin state guaranties normal homeostasis in the adult heart. Pathological stimuli leading to active pSer675-β-catenin accumulation activates Wnt signalling and the epigenetic state switches on to ‘active’, replaced by transcriptionally active pSer675-β-catenin bound to TCF7L2, leading to enriched H3K27ac occupancy and a high Wnt transcriptional activity. This results in the expression of disease-associated genes leading to adverse remodelling and heart failure.