Abstract
Background
Parathyroidectomy (PTX) is done in cases of secondary hyperparathyroidism from chronic kidney disease to improve renal osteodystrophy. Despite this widespread practice, clinical outcomes regarding the benefits of this procedure are still lacking. Most studies in the literature have opted to report the laboratory outcome instead. Our study aimed to evaluate the postoperative clinical course for patients who had undergone total PTX without autoimplantation.
Methods and results
All patients who underwent PTX between January 2010 and February 2014 in a tertiary referral center were included in this study and followed up for 12 months. Laboratory outcome parameters include various preoperative and postoperative serial measurements of laboratory parameters. Patients’ hospitalizations and mortality records post-PTX were also retrieved and recorded. In all, 90 patients were included in this study. The mean age was 48 ± 18 years. The majority of the patients (54.4%) were male and 90% were on hemodialysis. The mean duration of dialysis was 8.0 ± 5.0 years. Indications for PTX were symptomatic bone pain (95.6%), fractures (3.3%) and calciphylaxis (1.1%). Mean preoperative values for serum calcium, phosphate, alkaline phosphatase and intact parathyroid hormone (iPTH) were 2.40 ± 0.23mmol/L, 1.92 ± 0.51 mmol/L, 689.60 ± 708.50 U/L and 311.90 ± 171.94 pmol/L, respectively. The majority (92.2%) had all four glands removed and 92.2% of the glands showed hyperplasic changes. One year after PTX, 90 patients (100%) had serum iPTH <8 pmol/L and 28 patients (31%) had unmeasurable iPTH levels. A total of 15% of patients had hospitalizations for various reasons and of these, 50% were within 90 days. The mean hospital stay was 14.4 ± 18.6 days. The mortality rate was 4.4% and of these, 25% were in first 30 days. Causes of death were mainly from sepsis (75%) and acute coronary syndrome (25%). One patient (1.1%) had a relapse.
Conclusions
Even though PTX markedly reduces postoperative serum iPTH levels, it carries with it significant risk of morbidity and mortality.
Keywords: clinical course, dialysis, morbidity, mortality, postparathyroidectomy
Introduction
Secondary hyperparathyroidism (SHPT) is a frequently encountered problem in the management of patients with chronic kidney disease (CKD). SHPT has long been associated with poor patient outcomes, including accelerated atherosclerosis and metabolic bone disease [1, 2]. Parathyroidectomy (PTX) in end-stage renal failure (ESRF) is an effective method for normalization of calcium and parathyroid metabolism and for improving renal bone disease. Even though the initial management of SHPT involves medical treatment, ∼10% of patients will eventually require PTX [3]. In a study of a cohort of maintenance dialysis patients from the nationwide registry of the Japanese Society for Dialysis Therapy, patients who had undergone PTX had a 34% and 41% lower risk for all-cause and cardiovascular mortality, respectively [4]. Our center performs total PTX without autotransplantation to minimize the risk for recurrence and potential SHPT relapse. Despite the widespread practice of PTX, there is a scarcity of data describing the clinical outcomes that occur within 1 year after PTX. In this study we sought to describe the postoperative clinical course for 1 year in patients who had PTX without autoimplantation from January 2010 to February 2014.
Materials and methods
As per our national dialysis registry, 98% of our dialysis population received thrice weekly dialysis and the treatment duration was 4 h in 99% of the treatments. More than 90% of the patients were prescribed hemodialysis with a blood flow rate of >250 mL/min. In all, 86.8% of patients received dialysis through a functioning fistula. The mean and median delivered Kt/V were 1.5 and 1.4, respectively, for hemodialysis patients and 2.0 and 1.9 for those receiving peritoneal dialysis [5]. All patients who underwent PTX from January 2010 to February 2014 in a tertiary renal referral center (Serdang Hospital, Malaysia) were included and followed up for 12 months post-PTX. These patients were managed as per our published protocol during their hospitalization for PTX [6]. Table 1 describes the rate of infusion of calcium gluconate after surgery based on the serum alkaline phosphatase (ALP) level on admission. Preoperative and postoperative serial values of bone parameters such as calcium, phosphorus, ALP and intact parathyroid hormone (iPTH) were measured. Patients’ hospitalization and mortality records were retrieved from hospitals and dialysis centers via feedback letters and telephone calls to these centers. The clinical outcomes of interest were all-cause mortality and hospitalization events, including the reasons for admission, duration of stay and fracture rate post-PTX. The normal range of corrected calcium in this study is defined as between 2.1 and 2.6 mmol/L and any readings outside this range are defined as hypocalcemia and hypercalcemia, respectively. The study was approved by the ethics committee and registered under the National Medical Research Register of Malaysia.
Table 1.
Rate of infusion of calcium gluconate after surgery based on serum ALP level on admission
| ALP (IU/L) | Infusion rate (mL/h) | Elemental calcium (mg/day) | Dialysate calcium (mmol/L) |
|---|---|---|---|
| >700 | 40 | 3470 | 1.75 |
| 500–700 | 20 | 2429 | 1.75 |
| <500 | 10 | 1736 | 1.75 |
Statistical analysis
The characteristics of dialysis patients who underwent PTX were evaluated using mean and standard deviation (SD) for continuous variables and absolute number and percentage for categorical variables. SPSS version 23 (IBM, Armonk, NY, USA) was used for analysis. P-values <0.05 were considered to be statistically significant.
Results
We identified a total of 115 patients who underwent PTX between January 2010 and February 2014, of which 90 patients fulfilled the inclusion criteria. Patients with incomplete data or those who refused to provide consent were excluded. Table 2 describes the baseline characteristics of the PTX patients recruited into the study. The majority of the patients [n = 49 (54.4%)] were male with a mean age of 46.0 ± 18.0 years. Most patients [n = 81 (90%)] were doing hemodialysis at the time of PTX and the mean duration of dialysis was 8.0 ± 5.0 years. In all, 12 (13.3%) of the patients had diabetes mellitus. Most patients [n = 86 (95.6%)] had bone pain and 3 patients (3.3%) had fractures prior to PTX. The majority of patients [n = 83 (92.2%)] had all four parathyroid glands removed and histopathological findings showed hyperplasia in 83 patients (92.2%). Mean values of serum calcium, phosphate, albumin, ALP and iPTH prior to PTX were 2.40 ± 0.23 mmol/L, 1.92 ± 0.51 mmol/L, 689.60 ± 708.50 U/L and 311.90 ± 171.94 pmol/L, respectively. Table 3 compares of the baseline characteristics among included versus excluded PTX patients. The baseline characteristics were comparable.
Table 2.
Baseline characteristics of included parathyroidectomy patients (N = 90)
| Characteristics | n | % | Mean ± SD |
|---|---|---|---|
| Age (years)a | 46.0 (18.0) | ||
| Male (%) | 49 | 54.4 | |
| Duration of dialysis (years)a | 8.0 (5.0) | ||
| Hemodialysis (%) | 81 | 90.0 | |
| Primary disease (%) | |||
| Hypertension | 24 | 26.7 | |
| Glomerulonephritis | 13 | 14.4 | |
| Diabetes mellitus | 12 | 13.3 | |
| Unknown | 35 | 38.9 | |
| Othersb | 6 | 6.7 | |
| Symptoms (%) | |||
| Bone pain | 86 | 95.6 | |
| Fracture | 3 | 3.3 | |
| Calciphylaxis | 1 | 1.1 | |
| Operative findings (%) | |||
| All four glands removed | 83 | 92.2 | |
| Hyperplasia | 83 | 92.2 | |
| Adenoma | 7 | 7.8 | |
| Biochemical parameters | |||
| Calcium (mmol/L) | 2.40 ± 0.23 | ||
| Phosphate (mmol/L) | 1.92 ± 0.51 | ||
| Albumin (g/L) | 35.93 ± 5.27 | ||
| ALP (U/L)a | 689.60 (708.50) | ||
| IPTH (pmol/L) | 311.90 ± 171.94 |
Median (interquartile range).
Others—obstructive uropathy, etc.
Table 3.
Comparisons of baseline characteristics of all PTX patients (N = 115)
| Characteristics | Included | Excluded | P-value |
|---|---|---|---|
| (n = 90) | (n = 25) | ||
| Age (years)a | 46.0 (18.0) | 43.5 (15.0) | 0.776 |
| Male (%) | 54.4 | 46.2 | 0.456 |
| Duration of dialysis (years)a | 8.0 (5.0) | 8.0 (6.5) | 0.897 |
| Hemodialysis (%) | 90.0 | 100 | 0.093 |
| Primary disease (%) | |||
| Hypertension | 26.7 | 7.7 | 0.144 |
| Glomerulonephritis | 14.4 | 7.7 | |
| Diabetes mellitus | 13.3 | 26.9 | |
| Unknown | 38.9 | 50.0 | |
| Others | 6.7 | 7.7 | |
| Symptoms (%) | 0.550 | ||
| Bone pain | 95.6 | 100 | |
| Fracture | 3.3 | 0 | |
| Calciphylaxis | 1.1 | 0 | |
| Operative findings (%) | |||
| All four glands removed | 92.2 | 96.2 | 0.696 |
| Histopathology findings | |||
| Hyperplasia | 92.2 | 88.5 | 0.547 |
| Adenoma | 7.8 | 11.5 | 0.247 |
| Biochemical parameters | |||
| Calcium (mmol/L) | 2.40 ± 0.23 | 2.40 ± 0.28 | 0.727 |
| Phosphate (mmol/L) | 1.92 ± 0.51 | 1.67 ± 0.44 | 0.021 |
| Albumin (g/L) | 35.93 ± 5.27 | 41.69 ± 3.69 | 0.063 |
| ALP (U/L)a | 689.60 (708.50) | 483.50 (581.75) | 0.317 |
| IPTH (pmol/L) | 311.90 ± 171.94 | 231.47 ± 215.90 |
Median (interquartile range), tested using Mann–Whitney U.
One-year outcomes
Bone parameters
The iPTH level showed an abrupt reduction after PTX. By 1-year post-PTX, 90 patients (100%) had serum iPTH levels <8 pmol/L and 28 patients (31.1%) had undetectable levels. Figure 1 shows the mean ALP level over time after PTX. The mean ALP values show a gradual decrease and normalization by 1-year post-PTX. One patient had relapsed with hyperparathyroidism from the partially removed parathyroid glands and required a second exploratory PTX procedure.
Fig. 1.
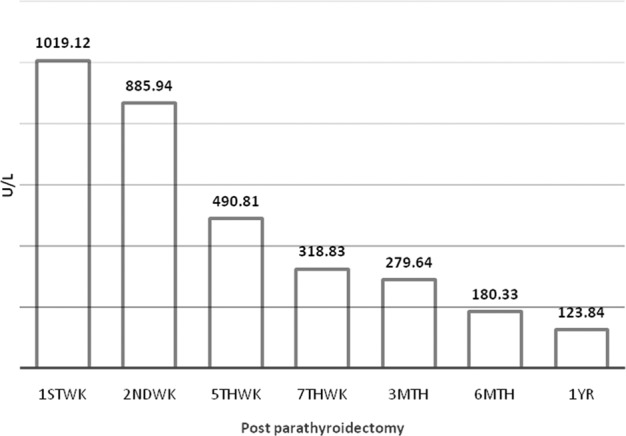
Mean ALP level post-PTX (N = 90).
Clinical course
Table 4 describes the clinical course post-PTX for 1 year. A total of 14 patients had hospitalizations within in 1 year of PTX for various reasons. There were 25 hospital admissions for these 14 patients. The 1-year hospitalization rate was 14%, of which 44% were within the first 90 days. The mean hospital stay was 14.4 ± 18.6 days. The reasons for hospitalization were hypo/hypercalcemia [n = 9 (36.0%)], sepsis [n = 6 (32%)] and fluid overload [n = 4 (16%)]. Hospitalization due to fracture was 4% (n = 1) post-PTX. There was no difference in baseline characteristics and biochemical profiles between the hospitalized and nonhospitalized patients (Table 5). The 1-year mortality among patients undergoing PTX was 4.4%, of which 25% were within the first 30 days. Acute coronary syndrome was noted in 25% of patients.
Table 4.
Clinical course post-PTX (N= 90)
| Events | N (%) |
|---|---|
| iPTH changes (pmol/L), n (%) | |
| By 1 year, level <8 | 90 (100) |
| By 1 year, unmeasurable level | 28 (31.1) |
| Mortality over 1 year, n (%) | 4 (4.4) |
| In first 30 days (%) | 25 |
| In first 90 days (%) | 75 |
| Causes of death (%) | |
| Sepsis | 75 |
| Acute coronary syndrome | 25 |
| Hospitalization, n (%) | 14 (15.6) |
| Total hospitalizations, n | 25 |
| Hospitalizations in 30 days, n (%) | 10 (40) |
| Hospitalizations in 90 days, n (%) | 11 (44) |
| Days of hospitalization (mean ± SD) | 14.4 ± 18.6 |
| Causes of hospitalization, n (%) | |
| Hypocalcemia | 5 (20) |
| Hypercalcemia | 4 (16) |
| Fluid overload | 4 (16) |
| Vascular access sepsis | 4 (16) |
| Other sepsis | 2 (8) |
| Other vascular access issues | 3 (12) |
| Fracture | 1 (4) |
| Stroke | 1 (4) |
| Acute coronary syndrome | 1 (4) |
Table 5.
Comparison of baseline characteristics of hospitalized patients post-PTX
| Characteristics | Hospitalized | Non-hospitalized | P-value |
|---|---|---|---|
| (n = 14) | (n = 76) | ||
| Age (years), mean ± SD | 43.9 ± 14.7 | 45.1 ± 12.2 | 0.136 |
| Male (%) | 28.6 | 59.2 | 0.034 |
| Duration of dialysis (years), mean ± SD | 8.9 ± 3.5 | 8.7 ± 3.8 | 0.470 |
| Hemodialysis (%) | 85.7 | 90.8 | 0.561 |
| Primary disease (%) | |||
| Hypertension | 28.6 | 26.3 | 0.693 |
| Glomerulonephritis | 21.4 | 13.2 | |
| Diabetes mellitus | 7.1 | 14.5 | |
| Unknown | 42.9 | 38.2 | |
| Others | 0 | 7.9 | |
| Biochemical parameters, mean ± SD | |||
| Calcium (mmol/L) | 2.43 ± 0.18 | 2.40 ± 0.24 | 0.105 |
| Phosphate (mmol/L) | 1.86 ± 0.49 | 1.93 ± 0.52 | 0.404 |
| Albumin (g/L) | 34.8 ± 6.5 | 36.1 ± 5.1 | 0.388 |
| ALP (U/L) | 777.1 (468.2) | 821.6 (541.8) | 0.885 |
| IPTH (pmol/L) | 421.1 ± 268.01 | 283.9 ± 129.5 | 0.298 |
Discussion
The Kidney Disease: Improving Global Outcomes (KDIGO) international guideline group recommends that patients with CKD Stages 3–5D with severe hyperparathyroidism who fail to respond to medical therapy should undergo PTX [7]. The first PTX for SHPT was performed by Fergusson in 1967. Total PTX without autoimplantation was initially not favored as a treatment for SHPT due to the initial concerns of subclinical hypoparathyroidism. However, Ljutic et al. [8] followed up a cohort of 43 patients with total PTX without autoimplantation and reported that nearly 8 years after PTX, there was no evidence of occurrence of subclinical hypoparathyroidism or adynamic bone disease. Currently there is a general consensus that total PTX without autotransplantation is comparable to subtotal PTX and total PTX with autoimplantation. The decision to undertake which procedure will depend on the skill of the surgeon and the hospital. Despite the best intention of our surgeon to carry out total PTX in all cases, in 17% of the cases the surgeon failed to locate and remove all glands intraoperatively. The accuracy can be improved via intraoperative PTH assays or Tc99m sestamibi scans, which are not available in our hospital.
Few studies that have focused primarily on the benefits of PTX have shown an improvement in laboratory parameters postoperatively [9–11]. However, there are few papers that describe clinical outcome after PTX. Similar to these studies, we found significant improvement in biochemical bone parameters after PTX. However, we observed several significant clinical outcomes in the first 30 days and up to 1 year after PTX. The most pronounced outcome was a high hospitalization rate of 14% per year, of which 40% happened within 30 days and 44% within 90 days of PTX for various reasons. The mortality rate was 4.4% per year, of which 25% were within 30 days of PTX. One large-scale, observational study from the United States Renal Database System (USRDS) showed a survival benefit post-PTX after 1 year compared with matched controls, with reported mortality rates of 10.4% versus 12.2%. However, the short-term 30-day mortality was elevated (3.1% versus 1.2%) [12]. A more recent study by Ishani et al. [13] using the same USRDS registry found that mortality at 30 days was 2.0% and at 1 year was 9.8%. In this study, our mortality rate was much lower. Our 30-day mortality was 1.1% and at 1 year it was 4.4%. Our improved mortality rate may be attributed to a smaller sample size, improved surgical techniques or more meticulous post-PTX care.
We also found that PTX is associated with significant morbidities. These are mainly related to hospitalizations for hypo/hypercalcemia issues. We are currently tightening our own protocol-driven postoperative care regime so that the readmission of patients due to hypo-/hypercalcemia complications can be further decreased. In addition, we found a lower fracture rate after PTX [n = 1 (1.1%)] as compared with before PTX [n = 3 (3.3%)]. The sole fracture that occured after PTX was attributed to a spontaneous vertebral compression fracture secondary to osteoporosis. Our finding is in keeping with the findings of Rudser et al. [14] who evaluated the risk of fractures after PTX in a nationwide cohort and demonstrated a lower risk compared with matched controls.
Our study has demonstrated that the procedure of PTX does come with small but significant risks of mortality and morbidity. Although PTX is still the treatment of choice in cases of severe SHPT, there may be an increased role for calcimimetics to be considered as nonoperative treatment for SHPT in high cardiac risk patients.
Our study should be interpreted in light of the following limitations. First, our study included only patients undergoing maintenance dialysis. The applicability of our results to other dialysis populations is unknown. Second, our sample size is relatively small and from a single center. We were unable to determine whether the adverse outcomes were due to the PTX procedure or the fact that ESRF patients have an increased morbidity while undergoing elective general surgery procedures [15].
Conclusions
We hope that with our reported clinical outcome after PTX will contribute to the understanding of the risks involved, assisting providers and patients in making informed decisions. These data provide essential evidence regarding adverse clinical outcomes related to surgical PTX, which should be recognized when considering this procedure as a treatment option for patients with severe SHPT.
Conflict of interest statement
None declared.
References
- 1. Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 2. Lim CTS, Yap XH, Chung KJ. et al. Predictor of cardiovascular risks in end stage renal failure patients on maintenance dialysis. Pak J Med Sci 2015; 31: 1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley RN, Li S, Liu J. et al. The fall and rise of parathyroidectomy in U.S. hemodialysis patients, 1992 to 2002. J Am Soc Nephrol 2005; 16: 210. [DOI] [PubMed] [Google Scholar]
- 4. Komaba H, Taniguchi M, Wada A. et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 2015; 88: 350–359 [DOI] [PubMed] [Google Scholar]
- 5. Goh BL, Ong LM, Lim YN.. 21st Report of the Malaysian Dialysis and Transplant Registry 2013. Kuala Lumpur: National Renal Registry, Malaysian Society of Nephrology, 2014 [Google Scholar]
- 6. Goh BL, Yudisthra MG, Hisham AN.. Alkaline phosphatase predicts calcium requirements after total parathyroidectomy in patients receiving dialysis. Br J Surg 2010; 97: 185–188 [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 113: S1–S130 [DOI] [PubMed] [Google Scholar]
- 8. Ljutic D, Cameron JS, Ogg CS. et al. Long-term follow up after total parathyroidectomy without parathyroid reimplantation in chronic renal failure. QJM 1994; 87: 685–692 [PubMed] [Google Scholar]
- 9. Trombetti A, Stoermann C, Robert JH. et al. Survival after parathyroidectomy in patients with end-stage renal disease and severe hyperparathyroidism. World J Surg 2007; 31: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 10. Gagné ER, Ureña P, Leite-Silva S. et al. Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients . J Am Soc Nephrol 1992; 3: 1008–1017 [DOI] [PubMed] [Google Scholar]
- 11. Neonakis E, Wheeler MH, Krishnan H. et al. Results of surgical treatment of renal hyperparathyroidism. Arch Surg 1995; 130: 643–648 [DOI] [PubMed] [Google Scholar]
- 12. Kestenbaum B, Andress DL, Schwartz SM. et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int 2004; 66: 2010–2016 [DOI] [PubMed] [Google Scholar]
- 13. Areef Ishani, Jiannong Liu, Wetmore James B. et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol 2015; 10: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudser KD, de Boer IH, Dooley A. et al. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 2007; 18: 2401–2407 [DOI] [PubMed] [Google Scholar]
- 15. Schneider CR, Cobb W, Patel S. et al. Elective surgery in patients with end stage renal disease: what’s the risk? Am Surg 2009; 75: 790–793 [DOI] [PubMed] [Google Scholar]


