Figure 4. Quantitative phosphoproteomic analyses identifies BCR as a novel DDR1 substrate.
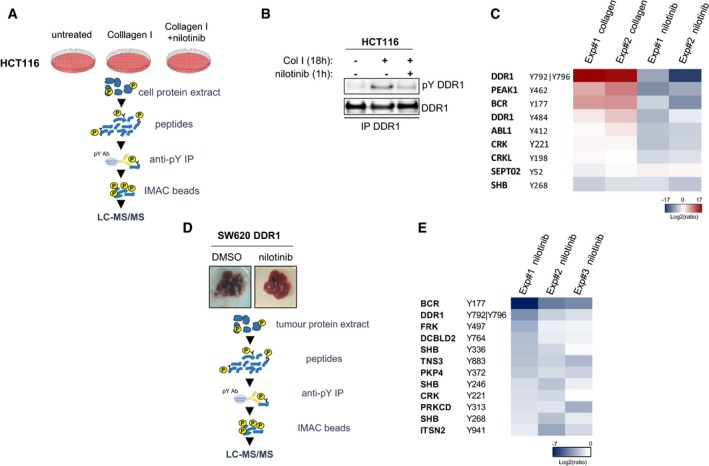
- Workflow of the phosphoproteomic analysis of the DDR1 kinase signalling cascade in HCT116 CRC cells.
- Western blot analysis of DDR1 tyrosine phosphorylation (pY) in HCT116 cells stimulated with 40 μg/ml collagen I for 18 h and incubated or not with 100 nM nilotinib for 1 h before cell lysis and immunoprecipitation (IP) with an anti‐DDR1 antibody.
- Heatmap of the phosphotyrosine peptides in HCT116 cellular extracts for which the level was consistently modulated upon collagen I stimulation and by nilotinib treatment (two biological replicates).
- Workflow of the phosphoproteomic analysis of the DDR1 kinase signalling cascade in liver metastases of the nude mice inoculated with DDR1 overexpressing SW620 cells described in Fig 3F.
- Heatmap of phosphotyrosine peptides in liver metastasis protein extracts (nude mice described in Fig 3F) that were consistently reduced upon nilotinib treatment (three biological replicates).
Source data are available online for this figure.
