Abstract
Research in maize is often performed using inbred lines that can be readily transformed, such as B104. However, because the B104 line flowers late, the kernels do not always mature before the end of the growing season, hampering routine seed yield evaluations of biotech traits introduced in B104 at many geographical locations. Therefore, we generated five hybrids by crossing B104 with the early‐flowering inbred lines CML91, F7, H99, Mo17, and W153R and showed in three consecutive years that the hybrid lines proved to be suitable to evaluate seed yield under field conditions in a temperate climate. By assessing the two main processes driving maize leaf growth, being rate of growth (leaf elongation rate or LER) and the duration of growth (leaf elongation duration or LED) in this panel of hybrids, we showed that leaf growth heterosis was mainly the result of increased LER and not or to a lesser extent of LED. Ectopic expression of the transgenes GA20‐oxidase (GA20‐OX) and PLASTOCHRON1 (PLA1), known to stimulate the LER and LED, respectively, in the hybrids showed that leaf length heterosis can be stimulated by increased LER, but not by LED, indicating that LER rather than LED is the target for enhancing leaf growth heterosis.
Keywords: GA20‐oxidase, heterosis, leaf growth, maize, plant biotechnology, PLASTOCHRON1
Short abstract
To enable transgenic maize research, hybrids between the inbred B104 that can be routinely transformed and early flowering inbreds were evaluated for yield components in three consecutive years. In addition, we show that leaf elongation rate is the main contributor to leaf growth heterosis in these hybrids, which can even be stimulated by overexpressing GA20OXIDASE, a known regulator of leaf elongation rate. Although leaf elongation duration has a limited contribution to the growth heterosis, the effect of the ectopic expression of PLASTOCHRON1, known to enhance leaf elongation duration and leaf growth, is still observed in the hybrids. This detailed understanding of the growth processes driving heterosis will be key to further breed for high yielding hybrids.
1. INTRODUCTION
Heterosis, also known as hybrid vigour, is the superior performance of F1 hybrid plants relative to their parental lines with regard to size, yield or stress tolerance (Shull, 1948), and is observed in various species, including cereals, vegetables, and flower crops (Fu et al., 2014). Despite the significant contribution of hybrid vigour to yield improvement in agriculture during the 20th century (Duvick, 2005), the basis of yield heterosis has thus far remained elusive.
Growth regulation is pivotal for many important agronomic traits that can display heterosis. The leaves of monocotyledonous plants, such as maize (Zea mays L.), are well suited to study organ growth, because the different cellular processes mediating its size increase, that is, cell division and cell expansion are largely physically separated. During steady‐state growth, the leaf base contains dividing cells, followed by a zone in which cells are exclusively expanding and a zone consisting of mature cells. Furthermore, in monocots, leaf growth can be monitored by taking daily leaf length measurements from leaf emergence until the leaf reaches its final size, allowing to calculate the leaf elongation rate (LER; Muller, Reymond, & Tardieu, 2001; Rymen, Coppens, Dhondt, Fiorani, & Beemster, 2010). Typically, leaf growth is maximal when the leaf appears outside the whorl of older leaves and maintains this growth rate for several days. This steady‐state period is followed by an exponential decline of growth until the final leaf size is reached. In addition to the LER, the period of leaf growth, which is described as the leaf elongation duration (LED), also contributes to the final leaf length (Voorend et al., 2014). The LER and LED are distinct processes, with no correlation at the phenotypic or molecular level in controlled conditions within two independent, recombinant inbred line populations (Baute et al., 2015; Baute et al., 2016). Several molecular components involved in the regulation of these growth mechanisms have been identified. Overexpression of GA20‐oxidase (GA20‐OX), encoding a rate‐limiting enzyme involved in gibberellin biosynthesis, enhances the LER by an increased number of dividing cells (Nelissen et al., 2012). On the other hand, ectopic expression of PLASTOCHRON1 (PLA1), encoding a P450 mono‐oxygenase, results in a longer growth period by maintaining maximal cell division for a longer time (Sun et al., 2017).
The maize B104 inbred line is a temperate Stiff Stalk line routinely used for generating transgenic lines (Anami et al., 2010; Coussens et al., 2012; B. R. Frame et al., 2006) and is closely related to B73 (K. Liu et al., 2003), for which the reference genome is available (Schnable et al., 2009). However, the late‐flowering B104 inbred line can have a limited or even no seed set in a maritime temperate climate (Voorend et al., 2016) and even in the corn belt (Sun et al., 2017), limiting its usefulness to study seed‐related traits. Therefore, we evaluated the performance of different B104 hybrids obtained after crossing with early‐flowering inbred lines to extend the capacity of B104 for testing transgenes introduced to enhance seed yield‐related traits. B104 was crossed with five distinct early‐flowering inbred lines from different heterotic groups: temperate non‐Stiff Stalk lines (H99, Mo17, and W153R) and mixed lines (CML91 and F7; K. Liu et al., 2003). These five hybrids and their parental lines were assessed in Belgian field conditions during three consecutive years (2013–2015) for multiple yield‐related traits. Based on the heterotic response over the different field trials (FTs), a distinction could be made between the hybrids B104xCML91, B104xF7, and B104xMo17 with respect to the stability of the heterotic response in variable environmental conditions as compared with B104xH99 and B104xW153R. In parallel to the FTs, the hybrids were also evaluated in controlled conditions using the maize leaf as a model for growth. Heterotic leaf growth was observed in all investigated hybrids and mainly resulted from an enhanced LER, whereas the LED only made a limited contribution. To determine if stimulating the LER or LED could enhance hybrid growth, the effect of constitutive GA20‐OX expression (Nelissen et al., 2012) and ectopic PLA1 expression (under the control of the GA2OX promoter; Sun et al., 2017) was assessed in the five B104 hybrids. All transgenic hybrids had longer leaves than the control hybrids showing the high expressivity of the transgenic constructs. Leaf length and LER heterosis could be stimulated, whereas LED heterosis could not.
2. RESULTS
2.1. Early‐flowering B104 hybrids allow the evaluation of seed yield in a temperate climate
The well‐studied B104 inbred line has been used in numerous studies to evaluate effects of transgenes (Char et al., 2015; Coussens et al., 2012; B. R. Frame et al., 2006; B. Frame, Main, Schick, & Wang, 2011; Ko et al., 2016; Kumar et al., 2015; Nahampun, López‐Arredondo, Xu, Herrera‐Estrella, & Wang, 2016; Nelissen et al., 2012; Nelissen et al., 2015; Sun et al., 2017). Because seed set of the late‐flowering B104 line is highly dependent on the growth season, there is no guarantee that seed‐related traits can be analysed in a temperate climate (Voorend et al., 2016). Therefore, we generated five B104 hybrid lines by crossing B104 and early‐flowering inbred lines (CML91, F7, H99, Mo17, and W153R), and evaluated these lines in a 3‐year FT in Belgium. Temperature and rainfall varied between the 3 years, with a cold spring in 2013, heavy rainfall in August 2014 and a limited rainfall in the summer of 2015 (Figure S1a–b).
For traits related to reproductive timing, the B104 hybrids closely resembled the early‐flowering inbreds (Figure S2), resulting in a good seed set in all 3 years. Conversely, the parental B104 inbred showed a delayed reproductive timing with no or few kernels in 2013 (Figures 1 and S2) owing to the cold spring and subsequent lower growing degree units (Figure S1c). In 2013, the B104 plants still did not show reproductive organs at 19 weeks after sowing (WAS), whereas the B104 hybrid lines already showed pollen shed and silk appearance before 19 WAS (starting at 16 WAS). These data show that the B104 hybrids never encountered problems in the 3‐year‐trial period with seed set in the Belgian climate in contrast to B104 plants.
Figure 1.

Seed set in the Belgian temperate climate of the B104 hybrid lines. Each picture displays a representative ear for B104, the F1 hybrid and the parental inbred line (from left to right). The scale bars represent 5 cm
Representative ears per genotype were studied in more detail for ear‐related traits (length, width, and weight) and kernel‐related traits (number of kernels per row, kernel row number, number of kernels per ear and 100‐kernel weight; Table S1). Heterosis for kernel‐ (Table S2) and ear‐related (Table S3) traits was expressed relative to the mid‐parent (mid‐parent heterosis; MPH) or the highest parent (high‐parent heterosis; HPH) value. Because the unfavourable conditions in 2013 resulted in no or a limited amount of kernels for several inbreds (Figure 1), the 2013 FT was excluded for the calculation of the heterotic effects on kernel‐related traits (Table S2). Kernel row number was the only kernel‐related trait not showing best‐parent heterosis (BPH) in any of the five hybrids (Table S2). For all other ear‐ and kernel‐related traits, the B104xCML91, B104xMo17, and B104xF7 hybrids showed BPH in all FTs (Tables S2 and S3), with the exception of B104xMo17 for the number of kernels per ear in 2015 and for the 100‐kernel weight in 2014, which did show significant MPH (Table S2). The ear‐ and kernel‐related traits in the B104xW153R and B104xH99 hybrids did not show BPH in at least one FT (Tables S2 and S3).
Thus, taking all three FTs into account, a distinction can be made between the hybrids based on their heterotic response. The B104xCML91, B104xF7, and B104xMo17 hybrids always displayed heterosis for ear‐ and kernel‐related traits, with in the majority of the cases BPH, whereas the B104xH99 and B104xW153R hybrids performed equally or worse relatively to the mid‐parent in at least one FT for the majority of the investigated traits. This suggests that the heterotic response for ear‐ and kernel‐related traits of the B104xCML91, B104xF7, and B104xMo17 hybrids was less sensitive to the environmental conditions than those of B104xH99 and B104xW153R.
2.2. The environment strongly influences the heterotic effect
To study the correlation between field and lab conditions, the final leaf length (FLL) of the fourth leaf was monitored in the FTs (2014–2015) and in the growth chamber (GC; Figure 2). The average FLL was about two to three times larger in the GC compared to the field (Table S4). This considerable difference in performance was present for both hybrid and parental inbred lines. In the GC, the hybrid lines displayed weaker heterosis levels for the FLL (2–14% MPH) compared to those of the FTs of 2014 (8–52% MPH) and 2015 (17–48% MPH; Table 1). All hybrids, including B104xH99 and B104xW153R, displayed heterosis in both FTs, as opposed to what was observed for the ear‐related traits (Table S3). The fact that the heterosis levels were reduced in the GC compared to the FTs indicates that the inbred lines were more sensitive to the environmental conditions in the field. In summary, leaf length heterosis levels in changing conditions of the FTs were in general higher than in well‐controlled lab conditions, whereas the absolute performance was higher in lab conditions.
Figure 2.
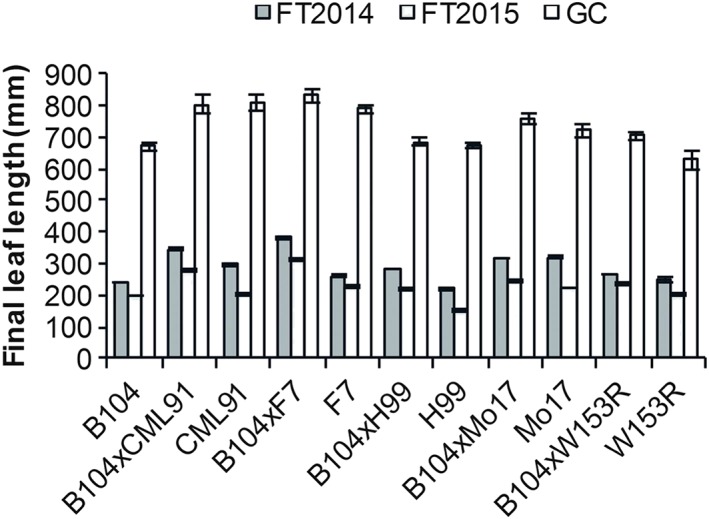
Final leaf length in field and controlled conditions. Final length of the fourth leaf (average ± standard error) for the field trial (FT) of 2014 (n = 41–162) and 2015 (n = 30–121) and in the growth chamber (GC; n = 3–10) for all hybrid and parental inbred lines
Table 1.
Heterotic effects on final leaf length in controlled conditions in a growth chamber (GC) and in field conditions (2014–2015)
| 2014 | 2015 | GC | ||||
|---|---|---|---|---|---|---|
| (%MPH) | (%BPH) | (%MPH) | (%BPH) | (%MPH) | (%BPH) | |
| B104xCML91 | 27.6** | 15.7** | 41.0** | 38.9** | 8.6* | −0.7 |
| B104xF7 | 52.2** | 47.3** | 47.7** | 38.2** | 13.8** | 5.1 |
| B104xH99 | 22.1** | 16.4** | 25.8** | 12.3** | 1.7 | 1.6 |
| B104xMo17 | 12.7** | −1.1 | 17.3** | 10.2** | 9.1** | 5.3 |
| B104xW153R | 8.4** | 7.8** | 20.1** | 19.4** | 8.3* | 5.0 |
Note. The %MPH and %BPH values refer to the average hybrid performance relative to the mid‐parent or best‐parent value, respectively.
MPH = mid‐parent heterosis; BPH = best‐parent heterosis.
Statistical significance (Wald test) is indicated by p < .01.
Statistical significance (Wald test) is indicated by p < .05.
2.3. Leaf elongation rate is the main driver of heterotic leaf growth in early‐flowering B104 hybrids in controlled conditions
To gain better insight into which processes are at the basis of leaf growth heterosis, the FLL, LER, and LED of the fourth leaf were determined for the B104 hybrids and their parental inbred lines in controlled conditions (Table S5). The inbred lines CML91, F7, and Mo17 had an increased FLL compared to B104, due to a combination of an enhanced LER and LED, or, in case of CML91, due to only an increased LER. The FLL of H99 and W153R was not significantly different from B104, but H99 and W153R had a significantly increased LED compared to B104, but no significant effect on the LER. These different contributions of the growth mechanisms towards leaf growth in the various parental inbred lines indicate that growth is regulated by diverse mechanisms in these lines. When leaf growth was compared between the parental inbred lines and B104 hybrids, a distinction for the FLL, LER, and LED could be made between CML91, F7, Mo17, and their B104 hybrids on the one hand, and between B104, H99, W153R, and their B104 hybrids on the other hand (Figure S3 and Table S5).
All hybrids showed MPH but no BPH for the FLL, except B104xH99, which showed no heterotic effect (Table 2). The hybrids B104xCML91, B104xF7, and B104xMo17 showed no significant difference with the mid‐parent for LED, suggesting that their increase in FLL could be mainly attributed to the significantly increased LER. In B104xH99 and B104xW153R, the positive heterotic effect on LER is counteracted by a negative effect on LED, resulting in a FLL comparable to the mid‐parent or in an increased FLL, respectively (Table 2). In conclusion, the higher LER is the driving force behind leaf growth heterosis in B104 hybrids, whereas LED makes no major positive contribution.
Table 2.
Heterotic effects on the growth‐related traits of the fourth leaf under control conditions
| Final leaf length | Leaf elongation rate | Leaf elongation duration | ||||
|---|---|---|---|---|---|---|
| (%MPH) | (%BPH) | (%MPH) | (%BPH) | (%MPH) | (%BPH) | |
| B104xCML91 | 8.6** | −0.7 | 15.2** | 4.1 | −4.2 | −6.1 |
| B104xF7 | 13.8** | 5.1 | 18.3** | 10.3* | −2.9 | −9.9 |
| B104xH99 | 1.7 | 1.6 | 10.5** | 10.0** | −10.5** | −14.7 |
| B104xMo17 | 9.1* | 5.3 | 17.9** | 11.5** | −4.5 | −11.1 |
| B104xW153R | 8.3* | 5.0 | 17.3** | 10.3* | −11.8** | −8.3*, [Link] |
Note. The %MPH and %BPH values refer to the average hybrid performance relative to the mid‐parent or best‐parent value, respectively.
MPH = mid‐parent heterosis; BPH = best‐parent heterosis.
Statistical significance (Wald test) is indicated by p < .01.
Statistical significance (Wald test) is indicated by p < .05.
2.4. Altered GA20‐OX expression in early‐flowering B104 hybrids enhances the heterotic effect on final leaf length, leaf elongation rate and division zone size
The LER was identified as the main driver of heterotic growth in the five B104 hybrids. To evaluate if additional stimulation of the LER could further improve hybrid growth, the GA20‐OX transgene (UBIL‐AtGA20‐OX in a B104 background) was introduced in the five different hybrid backgrounds, and the growth of their fourth leaf was evaluated in the GC (Table S6). Because the GA20‐OX line was reported to have an increased LER due to an enlarged size of the division zone (DZ; Nelissen et al., 2012), the DZ size was also measured in the hybrids and parents. Because the effect of GA20‐OX was very similar in the different independent lines (Nelissen et al., 2012), only the highest overexpressing line was chosen to make the hybrids.
The transgenic expression of GA20‐OX in all hybrids caused a significant increase in FLL (29–48%), LER (32–56%), and DZ size (20–39%) compared to their nontransgenic control hybrids (Table S7). The LED had a significant increase (6–18%) in the hybrid backgrounds B104xH99, B104xMo17, and B104xW153R. These findings are in line with the observations of GA20‐OX overexpression in B104, increasing leaf length by a higher LER, caused by a larger DZ size (Nelissen et al., 2012), and to a smaller extent by a longer LED (Voorend et al., 2014).
To examine if GA20‐OX overexpression had an effect on leaf growth heterosis, the heterotic responses in the GA20‐OX and control hybrids were analysed (Table 3 and Table S8). For the FLL, LER, and DZ size, all GA20‐OX hybrids showed BPH, except the LER for the GA20‐OXxW153R hybrid (Table 3). The heterotic effect of the B104 hybrids was enhanced in the GA20‐OX hybrids (Table 3), unless the B104 hybrid already displayed BPH. For the LED, no BPH was observed in either the B104 or GA20‐OX hybrids (Table 3). In conclusion, the presence of GA20‐OX can stimulate the heterotic response for the FLL, LER, and DZ size.
Table 3.
Best‐parent heterosis levels in GA20‐OX and control hybrids for leaf growth‐related traits
| B104 hybrid heterosis | GA20‐OX hybrid heterosis | |||||||
|---|---|---|---|---|---|---|---|---|
| FLL (%BPH) | LER (%BPH) | LED (%BPH) | DZ size (%BPH) | FLL (%BPH) | LER (%BPH) | LED (%BPH) | DZ size (%BPH) | |
| B104xCML91 | 1.4 | −8.8 | 4.0 | −6.9 | 35.7** | 30.7** | 1.9 | 12.5** |
| B104xF7 | 3.3 | 2.8 | 3.8 | 0.3 | 28.2** | 24.2** | −1.0 | 8.5** |
| B104xH99 | 12.1 | 13.4 | −7.8 | 5.3** | 9.6* | 10.7* | −1.5 | 16.8** |
| B104xMo17 | 34.7* | 38.0** | 3.9 | −10.6 | 21.5** | 16.3* | 12.1 | 23.4** |
| B104xW153R | 23.2** | 28.9** | −14.0 | 5.8** | 14.9** | 9.4 | −8.6 | 10.4** |
Note. The % BPH values refer to the average hybrid performance relative to the best‐parent value.
FLL = final leaf length; LER = leaf elongation rate; LED = leaf elongation duration; DZ = division zone; BPH = best‐parent heterosis.
Statistical significance (Wald test) is indicated by p < .01.
Statistical significance (Wald test) is indicated by p < .05.
2.5. Stimulating the LED by mild overexpression of PLA1 is able to increase leaf growth and enhance heterotic performance in early‐flowering B104 hybrids
The heterotic effect in B104 hybrids mainly works on the LER and not the LED. Previously, we have shown that the maize PLA1 gene, when expressed under control of the GA2OX promoter, enlarges the leaf size by extending the LED (Sun et al., 2017). We therefore evaluated if combining the processes of LER and LED could further stimulate leaf growth heterosis. To this end, we created hybrids between the GA2OX‐PLA1 transgenic line in a B104 background, and the five inbreds (Table S9). Of the three independent lines showing similar phenotypes (Sun et al., 2017), we selected line GA2OX‐PLA1_P2, for which to most detailed phenotypic characterization was available in B104, to make the hybrids.
Comparing the growth of the fourth leaf in PLA1‐overexpressing hybrids relative to control hybrids revealed a significant increase in the FLL (10–13%; Table S10). The LED was always positively increased albeit not statistically significant for the hybrids B104xCML91 and B104xH99. A positive effect was also detected for the LER in all backgrounds, but the effect was not statistically significant for B104xCML91 and B104xMo17. In conclusion, the effect of PLA1 overexpression on leaf growth was comparable between the different hybrid backgrounds, and a combination of both LER and LED appears to drive the growth enhancement by PLA1 overexpression in hybrid backgrounds.
In addition, the PLA1 line is known to positively affect leaf width (Sun et al., 2017). Examination of the five PLA1 hybrid lines showed a significantly increased leaf width (8–16%) and leaf area (21–49%) relative to the control hybrids, except for the leaf width in the B104xCML91 background (Table S10). Ectopic expression of PLA1 increased the observed heterotic effect on leaf width and area in all hybrids, except for B104xMo17, in which no heterotic effect was detected for both traits. Leaf area is a function of leaf length and width and the heterosis level for leaf area was approximately double of those for leaf length and width (Table S11).
In PLA1 hybrids, the BPH levels were enhanced for the FLL compared to the control hybrids, except for B104xH99 and B104xW153R (Table 4). Focusing on the growth processes LER and LED revealed that the BPH for the LER was enhanced in four of the PLA1 hybrids, whereas the LED only showed enhanced heterosis in the F7 hybrid (Table 4 and Table S12).
Table 4.
Best‐parent heterosis levels in PLA1 and control hybrids for growth‐related traits of the fourth leaf
| Heterosis B104 hybrid | Heterosis PLA1 hybrid | |||||
|---|---|---|---|---|---|---|
| FLL (%BPH) | LER (%BPH) | LED (%BPH) | FLL (%BPH) | LER (%BPH) | LED (%BPH) | |
| B104xCML91 | 10.0* | 24.2** | −9.8 | 24.0** | 38.1** | −7.1 |
| B104xF7 | 15.1** | 21.7** | −0.1 | 26.5** | 29.2** | 10.0** |
| B104xH99 | 13.3** | 13.5** | −5.6 | 6.9* | 9.7** | −2.4 |
| B104xMo17 | −2.7 | 3.8 | −2.1 | 6.8* | 8.3* | −1.3 |
| B104xW153R | 0.9 | 16.5** | −8.6 | 6.3 | 22.4** | −0.9 |
Note. The % BPH values refer to the average hybrid performance relative to the best‐parent value.
FLL = final leaf length; LER = leaf elongation rate; LED = leaf elongation duration; BPH = best‐parent heterosis.
Statistical significance is indicated by p < .01.
Statistical significance is indicated by p < .05.
Using the data of all transgenic experiments, the general combining ability (GCA) of B104 and the transgenic lines, GA20‐OX and PLA1, was assessed and compared for the traits FLL, LER, and LED (Table S13). The transgenic lines had a significantly higher GCA relative to B104 for the three analysed traits. Comparing the transgenic lines revealed that the GA20‐OX line had a significantly higher GCA for FLL and LER, but no significant difference was observed for LED compared to PLA1. Thus, the GA20‐OX line, known to affect LER, had the best combining ability for FLL and LER. Remarkably, the PLA1 line, known to affect LED, had no better combining ability relative to GA20‐OX for LED, but this process was also not observed to cause the heterosis.
3. DISCUSSION
3.1. Inbred lines as B104 are suitable genetic backgrounds for generating (hybrid) transgenic lines
To date many maize lines are still recalcitrant to genetic transformation, limiting the genotypes available for genetic transformation. Efforts are being made to overcome this restriction, for example by the expression of the Baby boom and Wuschel2 maize genes, extending the range of maize genotypes that can efficiently be transformed (Lowe et al., 2016). Over the past 15 years, the perferred genotype for maize transformation was the Hi‐II hybrid line, due to its efficient tissue culture (B. R. Frame et al., 2002; Vega, Yu, Kennon, Chen, & Zhang, 2008). However, several backcrosses to B73 are necessary and subsequent generations will not have the same genetic background because of the hybid nature of Hi‐II. Some inbred lines, such as B104, A188, and Ky21 were shown to be very promising genetic backgrounds for making transgenic lines to assess various quantitative traits (B. R. Frame et al., 2006; B. Frame et al., 2011; Ishida, Hiei, & Komari, 2007). An inbred line provides a stable genetic background, avoiding the need for backcrosses, speeding up the time needed to analyse the transgenic lines. B104 belongs to the same inbred group as B73 (Liu et al., 2003) and the genomes are very similar (Romay et al., 2013), but the molecular studies in B104 are now tremendously facilitated by the draft genome of B104 that is currently already available at MaizeGDB (https://www.maizegdb.org/). However, the sometimes poor performance of inbred lines can be a limitation towards field evaluations, which could render the use of inbreds less efficient for phenotypic analysis. This limitation can be bypassed by crossing the transgenic inbred line to another inbred to generate transgenic hybrids, allowing quantitative trait assessment during field trials. More recently, efforts have been made to extend the range of maize inbreds that can be readily transformed by the expression of the Baby boom and Wuschel2 maize genes (Lowe et al., 2016), which will allow to choose appropriate inbreds for analysis or to make transgenic or mutant hybrids.
Here, five hybrids were generated by crossing the readily transformable B104 line with the early‐flowering inbred lines CML91, F7, H99, Mo17, and W153R to gain a better understanding of hybrid growth and to utilize their potential early‐flowering properties for future field evaluations of transgenic lines. The ability to evaluate the effect of a transgene in a hybrid background enlarges its agronomic relevance, certainly when dealing with yield‐related traits. B104 hybrids with Mo17 and B97 have been reported to have a consistently high yield performance (Hallauer, Lamkey, & White, 1997). In line with this observation, our data demonstrated that all five investigated B104 hybrid lines were suited to evaluate biomass and seed‐related traits in Belgian field conditions.
3.2. Suboptimal environmental conditions differently affect growth and heterosis of early‐flowering B104 hybrids
Our analysis of heterosis for ear‐ and seed‐related traits and FLL over three consecutive FTs revealed variation in heterosis levels caused by a different sensitivity of the inbred and hybrid lines to the different environmental conditions occurring during these three growing seasons. Hybrids have been reported to be more tolerant to stresses, for example drought or higher planting density, and as such are more stable compared to inbred lines, positively affecting the observed grain yield heterosis levels (Araus, Sánchez, & Cabrera‐Bosquet, 2010; Betrán, Beck, Bänziger, & Edmeades, 2003a; W. Liu & Tollenaar, 2009). Although hybrids appear to be more efficient than inbreds in using the available resources under improved growing conditions (Munaro, Eyhérabide, D'Andrea, Cirilo, & Otegui, 2011), they are affected more than inbreds by nitrogen deficiency in the field (D'Andrea, Otegui, Cirilo, & Eyhérabide, 2009). These observations show that suboptimal conditions can both positively and negatively affect yield heterosis levels, depending on the differential sensitivity of the hybrid and parental lines to the environment, which is in line with our observations.
Our data show that environmental conditions differently affect heterosis levels for ear‐related traits in inbreds or hybrids due to different sensitivities, resulting in higher or lower heterosis levels, respectively. Stress conditions resulting in an overall reduced performance for ear‐related traits in both inbred and hybrid lines (e.g., FT of 2013) have a bigger impact on the inbred lines compared with the hybrid lines. The hybrids B104xH99 and B104xW153R showed no or a weak heterotic response for ear‐related traits in one of the FTs (2015), whereas the other hybrids (B104xCML91, B104xF7, and B104xMo17) showed heterotic effects in all FTs. Also in terms of absolute performance for the ear‐related traits, the hybrids B104xH99 and B104xW153R showed a decrease compared to the other three investigated hybrids (FT of 2015), whereas the hybrid performance in the other field trials showed a more or less similar range. Clearly, H99 and W153R are less well suited to generate stable, high‐performing hybrids with B104 inbred lines in the Belgian climate. The hybrid lines B104xCML91, B104xF7, and B104xMo17 performed well and showed heterosis in all investigated years, making them excellent candidate genotypes for transgenic research. The B104xCML91 hybrid ectopically expressing PLA1 has been successfully used to evaluate seed‐related traits under field conditions in Iowa and Belgium (Sun et al., 2017).
In addition to the distinction between the hybrids based on heterotic levels, the analysis of leaf growth revealed a negative contribution of the LED for the hybrids B104xH99 and B104xW153R, in contrast to the other hybrids. The H99 and W153R inbred lines both belong to the same heterotic subgroup (non‐Stiff Stalk‐mixed; K. Liu et al., 2003), while the other inbred lines belong to the heterotic (sub)groups Mixed (CML91 and F7) and non‐Stiff Stalk‐CO109:Mo17 (Mo17). The distinct performance of the B104xH99 and B104xW153R hybrids compared with the other hybrid lines in combination with their common genetic basis, may indicate the existence of a common regulation mechanism affecting growth processes in response to specific environmental conditions.
3.3. Heterosis is affecting multiple traits and growth mechanisms
Grain yield is among the most important and well‐studied traits in plant breeding. However, yield is a quantitative trait, which is affected by many genetic factors interacting with the environment, and the often unpredictable nature of these interactions prevents a straightforward understanding of yield heterosis. Heterosis is observed for multiple traits in maize, and the average heterosis levels differ largely between the various traits (Betrán, Beck, Bänziger, & Edmeades, 2003b; Flint‐Garcia, Buckler, Tiffin, Ersoz, & Springer, 2009; Tollenaar, Ahmadzadeh, & Lee, 2004).
Yield heterosis is mainly based on endpoint measurements, when the plant has been exposed to different conditions during the entire growing season. Studying heterosis levels over time revealed that for some features heterosis remained relatively stable over time, whereas for other traits (e.g., biomass) heterosis varied throughout the lifecycle (Edlich‐Muth, Muraya, Altmann, & Selbig, 2016; Tollenaar et al., 2004). Heterosis phenotypes can be detected as early as in the embryo or young seedling (Hoecker, Keller, Piepho, & Hochholdinger, 2006; Meyer, Pospisil, & Scholten, 2007). Our data showed heterosis for all hybrids on the early trait FLL, whereas at the end of the growing season, during which plants have been subjected to various environmental cues, a distinction could be made between two classes of hybrids based on their heterotic response for ear‐related traits in the FT of 2015. Kernel‐related traits assessed at the end of the growth season also differed in their heterotic response, for example, the trait “kernel row number” had no or limited heterosis, whereas the trait “kernels per row”displayed high levels of heterosis in general.
Previous studies have shown that the traits “plant yield” and “total kernel weight” had the highest heterosis levels with hybrid performance exceeding the double of the best‐parent value, whereas most observed traits only exhibited 10–30% BPH (Flint‐Garcia et al., 2009). Multiplicative traits as plant yield and total kernel weight are hypothesized to combine the variation from several other traits as plant height or ear length (Flint‐Garcia et al., 2009; Lippman & Zamir, 2007). We observed that heterosis levels in traits at the whole‐organ level such as leaf area were higher compared with their subtraits such as leaf width and FLL. Focusing on the trait FLL demonstrated that of the involved growth processes LER and LED, mainly LER contributed to FLL heterosis. The lower heterosis levels of subtraits could complicate more in‐depth research, while still a lot remains to be elucidated about the underlying growth mechanisms of yield heterosis.
3.4. Leaf elongation rate is the growth process stimulating growth in B104 hybrids in controlled conditions
The processes LER and LED have previously been reported to independently contribute to leaf size and no common molecular basis was found (Baute et al., 2015; Baute et al., 2016). Furthermore, high LER indicates a high biomass (Baute et al., 2015). Here, we demonstrate that in all five hybrids, the leaf growth heterosis resulted from an increased LER as compared with the LER in the parental inbred lines. In addition, increasing the LER by GA20‐OX overexpression could further enhance leaf length heterosis. These observations indicate that the LER is a robust mechanism in controlled conditions underlying leaf size heterosis, and understanding the molecular basis of the effect is an interesting goal for future research. On the other hand, LED also contributes to the FLL (Baute et al., 2016), but it made no major positive contribution to leaf growth heterosis in controlled conditions. In addition, increasing LED by PLA1 overexpression was insufficient to enhance LED heterosis in most hybrid lines.
3.5. The function of gibberellin in (leaf) growth heterosis
Gibberellin (GA) was previously hypothesized to play a role in heterosis for shoot growth because of the high concentrations of bioactive GA in hybrid versus inbred lines (Rood, Buzzell, Mander, Pearce, & Pharis, 1988). In addition, the maize GA biosynthesis genes GA20‐OX and GA3‐OX show high expression levels in the B73 × Mo17 hybrid, exceeding both parental lines, in mature leaves (Song et al., 2016). A previous study has shown that hybrids with the knock‐out dwarf1 mutation, which reduces the bioactive GA levels, still showed a heterotic response for traits as leaf width and leaf length, indicating that high GA levels are not needed for these heterotic responses (Auger, Peters, & Birchler, 2005). Alternatively, our study demonstrated that stimulating GA biosynthesis can make a positive contribution to the heterotic response for FLL, LER, and DZ size. The transgene appears to be able to boost heterotic traits and thus does more than simply conserve its growth effect in the different lines. Thus, genes identified in relation to growth heterosis have the potential to further boost hybrid growth and heterosis levels when modified.
4. EXPERIMENTAL PROCEDURES
4.1. Plant material and growth conditions
B104 hybrid seeds were derived by crossing inbred lines CML91, Mo17, H99, F7, and W153R as male plants with B104 plants. For the PLA1 and GA20‐OX hybrid experiments, hemizygous pGA2OX‐PLA1‐P2 (Sun et al., 2017) or homozygous UBIL‐AtGA20‐OX plants (Nelissen et al., 2012; Voorend et al., 2016) in a B104 background were crossed with the inbred lines. As control, B104 plants originating from the segregating transgenic lines were used. The inbred and transgenic lines used to generate hybrid seeds were used as parental lines in the experiments.
The growth chamber has a controlled relative humidity (55%), temperature (24°C), and light intensity (170 mmol m‐2 s‐1 photosynthetically active radiation at plant level) in a 16‐hr/8‐hr day/night rhythm provided by a combination of high‐pressure sodium vapour (RNP‐T/LR/400 W/S/230/E40; Radium) and metal halide lamps with quartz burners (HRI‐BT/400 W/D230/E40; Radium).
4.2. Field trials
The seeds were sown for 3 years (planting dates 25 April 2013, 16 May 2014, and 12 May 2015) in three independent sites in Belgium: two sites in Merelbeke (2013: 50°98′63.64″N, 3°78′64.84”O, 2014: 50°97′96.68″N, 3°78′12.40”O) and one in Zwijnaarde (2015: 51°00′96.06″N, 3°71′57.78”O). The planting scheme consisted of two randomized blocks containing one row per genotype or in case of the field trial from 2015 one randomized block with two adjacent rows per genotype. Commercial hybrids (2013: Ronaldinio, 2014: LOGO, and 2015: Ricardinio) were used as border plants surrounding the field trial. The sowing density was approximate 133.333 plants per hectare in 2013 and 2014 and 177.778 plants per hectare in 2015. The final plant density was on average 89.000, 88.500, and 94.000 plants per hectare, for 2013, 2014, and 2015 respectively. The FTs were completed (determination of final growth parameters) on 23 October 2013, 22 October 2014, and 14 October 2015. Temperature and rainfall were determined in the weather station at Merelbeke (50°59′06.97″N, 3°46′16.64”O). The growing degree units (GDU) were calculated according to the following formula: GDU = (Tmax + Tmin)/2 – Tbase. Before entering temperature data into the equation, Tmax and Tmin were set equal to Tbase if less than Tbase (=10 °C) and equal to Tupper threshold when greater than Tupper threshold (Tupper threshold = 30 °C; Viña et al., 2004).
4.3. Phenotypic analyses
The final length of the fourth leaf was measured from soil level to leaf tip when it was fully grown early in the growth season. In the field, the reproductive timing (appearance of tassel, ear, pollen, and silks for minimum 50% of the plants) was monitored on a weekly basis. At the end of the growth season, final growth parameters were determined. For the ear‐related traits, representative ears were randomly selected for each genotype while ensuring that the selected ears were no extreme outliers. The ears were dried for 4 days at 30 °C before the following traits were analysed: ear length, ear width, kernel row number, number of kernels per row, and weight. For each ear also the weight of 100 kernels and the number of kernels [100*(weight of all kernels)/weight of 100 kernels] was determined.
In the growth chamber, phenotyping of the fourth leaf occurred by measuring its length on a daily basis. The LER was the average rate of leaf elongation the first 5 days after leaf appearance and the LED was the period from 100 mm till the end of growth (Voorend et al., 2014). At the end of growth, the leaf blade was scanned and processed using ImageJ to determine the lamina area and maximum width. Division zone measurements were performed as previously described (Rymen et al., 2010).
4.4. Statistical analysis
In the case of two rows per genotype, the data of both rows was combined in the analysis. A Student's t‐test is used for pairwise comparisons, for example, comparing measurements of transgenic and control plants. p‐values below .05 were considered statistically significant. Statistical analysis for estimation of heterosis was performed by fitting a general linear model to the data using the proc glm procedure in SAS (Version 9.4 of the SAS System for windows 7 64bit. Copyright © 2002–2012 SAS Institute Inc. Cary, NC, USA, http://www.sas.com) and performing Wald statistics at the 5% significance level. MPH values were calculated using the formula MPH = [(F1 – MP)/MP]*100, where F1 = F1 hybrid value and MP = mid‐parent value [(P1 + P2)/2]. To test for significance of MPH values, the contrast F1 – MP was used. BPH values were calculated using the formula BPH = [(F1 – BP)/(BP)]*100, where BP = best‐parent value (P1 or P2). To test for significance of BPH values, the contrast F1 – BP was used.
To estimate the variance components of the tester crosses the following linear mixed model was fitted to the data, including both hybrid and parental genotypes: yijk = μ + gcai + gcaj + scaij + expk + error partitioning the phenotypic variation into random GCA effects of the i‐th tester and j‐th inbred line (i = 1…3: B104, GA20‐OX, PLA1; j = 1…5: CML91, F7, H99, Mo17, W153R) parameterized as a two separate matrices of indicator variables for the parents, random specific combining abilities (SCA) effects for the cross between the i‐th tester and the j‐th inbred line (i ≠ j), and random experiment effects. GCA and SCA effects were assumed to be normally and independently distributed with means zero and variance σGCA2 and σSCA2, respectively. Best linear unbiased predictors estimates for the GCA and SCA effects were generated together with an estimate for the standard error of differences between the estimated parameters of the GCA terms. Test statistics, that is, difference in best linear unbiased predictors divided by the corresponding standard error of differences, were calculated for comparisons between transgenic testers and B104, and all pairwise comparisons between the nontransgenic inbred lines. These ratios were supposed to follow approximately a t‐distribution with the df equal to the df of the error term in the model. p‐values were calculated on the t‐approximation to the test statistics for the contrasts. All analyses were performed using Genstat v18 (VSN International (2015) Genstat Reference Manual (Release 18), Part 3 Procedures. VSN International, Hemel Hempstead, UK).
CONFLICT OF INTEREST
No conflicts of interest are to be declared.
Supporting information
Figure S1. Environmental conditions during the field trials
Figure S2. Reproductive timing during the field trials
Figure S3. Leaf growth‐related traits of the B104 hybrids and their parental lines grown in controlled conditions
Table S1. Phenotypic data for ear‐ and kernel‐related traits in field conditions
Table S2. Heterotic effects on kernel‐related traits in field conditions (2014–2015)
Table S3. Heterotic effects on ear‐related traits in field conditions (2013–2015)
Table S4. Phenotypic data for final leaf length
Table S5. Leaf growth‐related traits of the B104 hybrids and their parental lines grown in controlled conditions
Table S6. Phenotypic data for the GA20‐OX hybrid experiments
Table S7. Differences in leaf growth‐related traits of the fourth leaf between GA20‐OX and control hybrid plants grown under controlled conditions
Table S8. Mid‐parent heterosis levels in GA20‐OX and control hybrids for leaf growth‐related traits
Table S9. Phenotypic data for the PLA1 hybrid experiments
Table S10. Differences in leaf growth‐related traits of the fourth leaf between PLA1‐overexpressing and control hybrid plants grown under controlled conditions
Table S11 Heterotic effects on leaf width and leaf area of the fourth leaf in PLA1 hybrids under controlled conditions
Table S12. Mid‐parent heterosis levels in PLA1 and control hybrids for leaf growth‐related traits
Table S13. General combining ability of the B104, GA20‐OX and PLA1 lines for leaf growth‐related traits
ACKNOWLEDGMENTS
We thank all field trial volunteers, Dr. Véronique Storme, Dr. Marnik Vuylsteke, and Dr. Dorota Herman for statistical help, and Dr. Annick Bleys for help in preparing the manuscript. This research was supported by grants from the European Research Council under the European Community's Seventh Framework Program [FP7/2007‐2013] under ERC Grant agreement [339341‐AMAIZE]11, from Ghent University (“Bijzonder Onderzoeksfonds Methusalem Project” BOF08/01 M00408 and Multidisciplinary Research Partnership “Biotechnology for a Sustainable Economy” Grant 01MR0510W), and from the Interuniversity Attraction Poles Program (IUAP P7/29 “MARS”) initiated by the Belgian Science Policy Office.
Feys K, Demuynck K, De Block J, et al. Growth rate rather than growth duration drives growth heterosis in maize B104 hybrids. Plant Cell Environ. 2018;41:374–382. https://doi.org/10.1111/pce.13099
REFERENCES
- Anami, S. E. , Mgutu, A. J. , Taracha, C. , Coussens, G. , Karimi, M. , Hilson, P. , … Machuka, J. (2010). Somatic embryogenesis and plant regeneration of tropical maize genotypes. Plant Cell, Tissue and Organ Culture, 102, 285–295. [Google Scholar]
- Araus, J. L. , Sánchez, C. , & Cabrera‐Bosquet, L. (2010). Is heterosis in maize mediated through better water use? The New Phytologist, 187, 392–406. [DOI] [PubMed] [Google Scholar]
- Auger, D. L. , Peters, E. M. , & Birchler, J. A. (2005). A genetic test of bioactive gibberellins as regulators of heterosis in maize. The Journal of Heredity, 96, 614–617. [DOI] [PubMed] [Google Scholar]
- Baute, J. , Herman, D. , Coppens, F. , De Block, J. , Slabbinck, B. , Dell'Acqua, M. , … Inzé, D. (2016). Combined large‐scale phenotyping and transcriptomics in maize reveals a robust growth regulatory network. Plant Physiology, 170, 1848–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baute, J. , Herman, D. , Coppens, F. , De Block, J. , Slabbinck, B. , Dell'Acqua, M. , … Inzé, D. (2015). Correlation analysis of the transcriptome of growing leaves with mature leaf parameters in a maize RIL population. Genome Biology, 16, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betrán, F. J. , Beck, D. , Bänziger, M. , & Edmeades, G. O. (2003a). Genetic analysis of inbred and hybrid grain yield under stress and nonstress environments in tropical maize. Crop Science, 43, 807–817. [Google Scholar]
- Betrán, F. J. , Beck, D. , Bänziger, M. , & Edmeades, G. O. (2003b). Secondary traits in parental inbreds and hybrids under stress and non‐stress environments in tropical maize. Field Crops Research, 83, 51–65. [Google Scholar]
- Char, S. N. , Unger‐Wallace, E. , Frame, B. , Briggs, S. A. , Main, M. , Spalding, M. H. , … Yang, B. (2015). Heritable site‐specific mutagenesis using TALENs in maize. Plant Biotechnology Journal, 13, 1002–1010. [DOI] [PubMed] [Google Scholar]
- Coussens, G. , Aesaert, S. , Verelst, W. , Demeulenaere, M. , De Buck, S. , Njuguna, E. , … Van Lijsebettens, M. (2012). Brachypodium distachyon promoters as efficient building blocks for transgenic research in maize. Journal of Experimental Botany, 63, 4263–4273. [DOI] [PubMed] [Google Scholar]
- D'Andrea, K. E. , Otegui, M. E. , Cirilo, A. G. , & Eyhérabide, G. H. (2009). Ecophysiological traits in maize hybrids and their parental inbred lines: Phenotyping of responses to contrasting nitrogen supply levels. Field Crops Research, 114, 147–158. [Google Scholar]
- Duvick, D. N. (2005). Genetic progress in yield of United States maize (Zea mays L.). Maydica, 50, 193–202. [Google Scholar]
- Edlich‐Muth, C. , Muraya, M. M. , Altmann, T. , & Selbig, J. (2016). Phenomic prediction of maize hybrids. Biosystems, 146, 102–109. [DOI] [PubMed] [Google Scholar]
- Flint‐Garcia, S. A. , Buckler, E. S. , Tiffin, P. , Ersoz, E. , & Springer, N. M. (2009). Heterosis is prevalent for multiple traits in diverse maize germplasm. PLoS One, 4, e7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, B. , Main, M. , Schick, R. , & Wang, K. (2011). Genetic transformation using maize immature zygotic embryos. Methods in Molecular Biology, 710, 327–341. [DOI] [PubMed] [Google Scholar]
- Frame, B. R. , McMurray, J. M. , Fonger, T. M. , Main, M. L. , Taylor, K. W. , Torney, F. J. , … Wang, K. (2006). Improved Agrobacterium‐mediated transformation of three maize inbred lines using MS salts. Plant Cell Reports, 25, 1024–1034. [DOI] [PubMed] [Google Scholar]
- Frame, B. R. , Shou, H. , Chikwamba, R. K. , Zhang, Z. , Xiang, C. , Fonger, T. M. , … Wang, K. (2002). Agrobacterium tumefaciens‐mediated transformation of maize embryos using a standard binary vector system. Plant Physiology, 129, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, D. , Xiao, M. , Hayward, A. , Fu, Y. , Liu, G. , Jiang, G. , & Zhang, H. (2014). Utilization of crop heterosis: A review. Euphytica, 197, 161–173. [Google Scholar]
- Hallauer, A. R. , Lamkey, K. R. , & White, P. R. (1997). Registration of five inbred lines of maize: B102, B103, B104, B105, and B106. Crop Science, 37, 1405–1406. [Google Scholar]
- Hoecker, N. , Keller, B. , Piepho, H.‐P. , & Hochholdinger, F. (2006). Manifestation of heterosis during early maize (Zea mays L.) root development. Theoretical and Applied Genetics, 112, 421–429. [DOI] [PubMed] [Google Scholar]
- Ishida, Y. , Hiei, Y. , & Komari, T. (2007). Agrobacterium‐mediated transformation of maize. Nature Protocols, 2, 1614–1621. [DOI] [PubMed] [Google Scholar]
- Ko, D. K. , Rohozinski, D. , Song, Q. , Taylor, S. H. , Juenger, T. E. , Harmon, F. G. , & Chen, Z. J. (2016). Temporal shift of circadian‐mediated gene expression and carbon fixation contributes to biomass heterosis in maize hybrids. PLoS Genetics, 12, e1006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , AlAbed, D. , Whitteck, J. T. , Chen, W. , Bennett, S. , Asberry, A. , … Gupta, M. (2015). A combinatorial bidirectional and bicistronic approach for coordinated multi‐gene expression in corn. Plant Molecular Biology, 87, 341–353. [DOI] [PubMed] [Google Scholar]
- Lippman, Z. B. , & Zamir, D. (2007). Heterosis: Revisiting the magic. Trends in Genetics, 23, 60–66. [DOI] [PubMed] [Google Scholar]
- Liu, K. , Goodman, M. , Muse, S. , Smith, J. S. , Buckler, E. , & Doebley, J. (2003). Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics, 165, 2117–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , & Tollenaar, M. (2009). Response of yield heterosis to increasing plant density in maize. Crop Science, 49, 1807–1816. [Google Scholar]
- Lowe, K. , Wu, E. , Wang, N. , Hoerster, G. , Hastings, C. , Cho, M.‐J. , … Gordon‐Kamm, W. (2016). Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell, 28, 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, S. , Pospisil, H. , & Scholten, S. (2007). Heterosis associated gene expression in maize embryos 6 days after fertilization exhibits additive, dominant and overdominant pattern. Plant Molecular Biology, 63, 381–391. [DOI] [PubMed] [Google Scholar]
- Muller, B. , Reymond, M. , & Tardieu, F. (2001). The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady‐state elongation and the establishment of the elongation zone. Journal of Experimental Botany, 52, 1259–1268. [PubMed] [Google Scholar]
- Munaro, E. M. , Eyhérabide, G. H. , D'Andrea, K. E. , Cirilo, A. G. , & Otegui, M. E. (2011). Heterosis × environment interaction in maize: What drives heterosis for grain yield? Field Crop . Res., 124, 441–449. [Google Scholar]
- Nahampun, H. N. , López‐Arredondo, D. , Xu, X. , Herrera‐Estrella, L. , & Wang, K. (2016). Assessment of ptxD gene as an alternative selectable marker for Agrobacterium‐mediated maize transformation. Plant Cell Reports, 35, 1121–1132. [DOI] [PubMed] [Google Scholar]
- Nelissen, H. , Eeckhout, D. , Demuynck, K. , Persiau, G. , Walton, A. , van Bel, M. , … De Jaeger, G. (2015). Dynamic changes in ANGUSTIFOLIA3 complex composition reveal a growth regulatory mechanism in the maize leaf. Plant Cell, 27, 1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen, H. , Rymen, B. , Jikumaru, Y. , Demuynck, K. , Van Lijsebettens, M. , Kamiya, Y. , … Beemster, G. T. S. (2012). A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Current Biology, 22, 1183–1187. [DOI] [PubMed] [Google Scholar]
- Romay, M. C. , Millard, M. J. , Glaubitz, J. C. , Peiffer, J. A. , Swarts, K. L. , Casstevens, T. M. , … Gardner, C. A. (2013). Comprehensive genotyping of the USA national maize inbred seed bank. Genome Biology, 14, R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood, S. B. , Buzzell, R. I. , Mander, L. N. , Pearce, D. , & Pharis, R. P. (1988). Gibberellins: A phytohormonal basis for heterosis in maize. Science, 241, 1216–1218. [DOI] [PubMed] [Google Scholar]
- Rymen, B. , Coppens, F. , Dhondt, S. , Fiorani, F. , & Beemster, G. T. S. (2010). Kinematic analysis of cell division and expansion. Methods in Molecular Biology, 655, 203–227. [DOI] [PubMed] [Google Scholar]
- Schnable, P. S. , Ware, D. , Fulton, R. S. , Stein, J. C. , Wei, F. , Pasternak, S. , … Wilson, R. K. (2009). The B73 maize genome: Complexity, diversity, and dynamics. Science, 326, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Shull, G. H. (1948). What is “heterosis”? Genetics, 33, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Zhang, Z. , Tan, X. , Jiang, Y. , Gao, J. , Lin, L. , … Kuai, B. (2016). Association of the molecular regulation of ear leaf senescence/stress response and photosynthesis/metabolism with heterosis at the reproductive stage in maize. Scientific Reports, 6, 29843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Cahill, J. , Van Hautegem, T. , Feys, K. , Whipple, C. , Novák, O. , … Nelissen, H. (2017). Altered expression of maize PLASTOCHRON1 enhances biomass and seed yield by extending cell division duration. Nature Communications, 8, 14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar, M. , Ahmadzadeh, A. , & Lee, E. A. (2004). Physiological basis of heterosis for grain yield in maize. Crop Science, 44, 2086–2094. [Google Scholar]
- Vega, J. M. , Yu, W. , Kennon, A. R. , Chen, X. , & Zhang, Z. J. (2008). Improvement of Agrobacterium‐mediated transformation in Hi‐II maize (Zea mays) using standard binary vectors. Plant Cell Reports, 27, 297–305. [DOI] [PubMed] [Google Scholar]
- Viña, A. , Gitelson, A. A. , Rundquist, D. C. , Keydan, G. , Leavitt, B. , & Schepers, J. (2004). Monitoring maize (Zea mays L.) phenology with remote sensing. Agronomy Journal, 96, 1139–1147. [Google Scholar]
- Voorend, W. , Lootens, P. , Nelissen, H. , Roldán‐Ruiz, I. , Inzé, D. , & Muylle, H. (2014). LEAF‐E: A tool to analyze grass leaf growth using function fitting. Plant Methods, 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorend, W. , Nelissen, H. , Vanholme, R. , De Vliegher, A. , Van Breusegem, F. , Boerjan, W. , … Inzé, D. (2016). Overexpression of GA20‐OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnology Journal, 14, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Environmental conditions during the field trials
Figure S2. Reproductive timing during the field trials
Figure S3. Leaf growth‐related traits of the B104 hybrids and their parental lines grown in controlled conditions
Table S1. Phenotypic data for ear‐ and kernel‐related traits in field conditions
Table S2. Heterotic effects on kernel‐related traits in field conditions (2014–2015)
Table S3. Heterotic effects on ear‐related traits in field conditions (2013–2015)
Table S4. Phenotypic data for final leaf length
Table S5. Leaf growth‐related traits of the B104 hybrids and their parental lines grown in controlled conditions
Table S6. Phenotypic data for the GA20‐OX hybrid experiments
Table S7. Differences in leaf growth‐related traits of the fourth leaf between GA20‐OX and control hybrid plants grown under controlled conditions
Table S8. Mid‐parent heterosis levels in GA20‐OX and control hybrids for leaf growth‐related traits
Table S9. Phenotypic data for the PLA1 hybrid experiments
Table S10. Differences in leaf growth‐related traits of the fourth leaf between PLA1‐overexpressing and control hybrid plants grown under controlled conditions
Table S11 Heterotic effects on leaf width and leaf area of the fourth leaf in PLA1 hybrids under controlled conditions
Table S12. Mid‐parent heterosis levels in PLA1 and control hybrids for leaf growth‐related traits
Table S13. General combining ability of the B104, GA20‐OX and PLA1 lines for leaf growth‐related traits


