Figure 5. The scaffold protein APPL1 facilitates HDAC3 regulation of AKT .
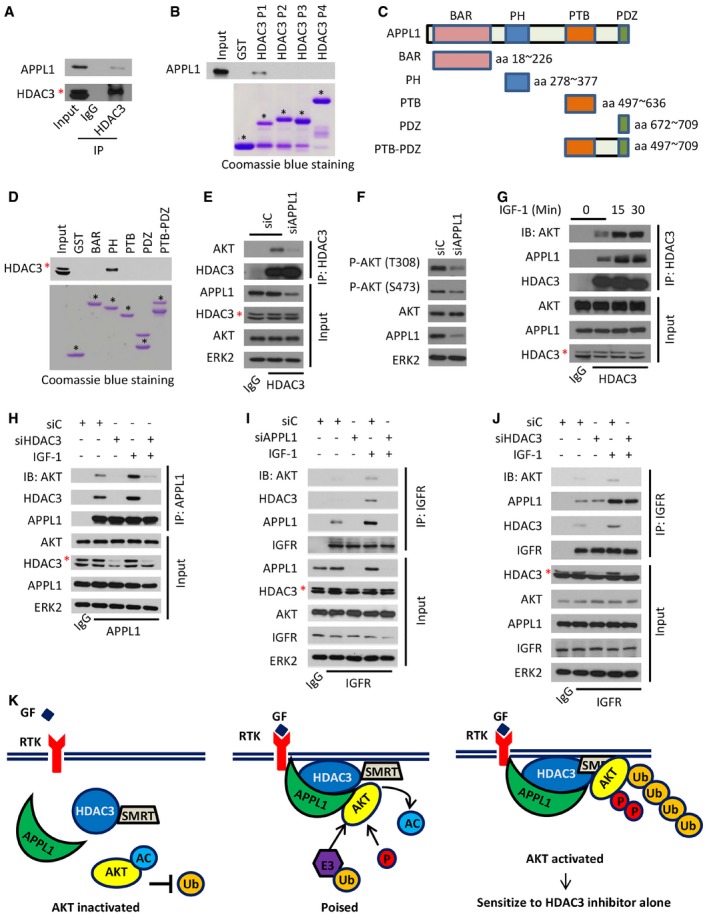
-
AC4‐2 cell lysate was prepared for IP and Western blots with the indicated antibodies. The asterisk (*) indicates the specific HDAC3 protein band.
-
BC4‐2 cell lysate was prepared for GST pull‐down assay using GST or GST‐HDAC3 recombinant proteins (stained with Coomassie blue, low panel) followed by Western blot with anti‐APPL1 antibody (upper panel). GST or GST‐HDAC3 recombinant proteins with expected molecular mass are indicated by asterisks.
-
CAn illustration depicts four functional domains (BAR, PH, PTB, and PDZ) of APPL1 used for construction of GST‐APPL1 recombinant proteins.
-
DC4‐2 cell lysate was prepared for GST pull‐down assay using GST or GST‐APPL1 recombinant proteins (stained with Coomassie blue, low panel) followed by Western blot with anti‐HDAC3 antibody (upper panel). The red asterisk (*) indicates the specific HDAC3 protein band, while the black ones indicate the specific domains of APPL1.
-
E, FC4‐2 cells were transfected with a pool of control or APPL1‐specific siRNAs for 48 h followed by IP and Western blots with the indicated antibodies. The asterisk (*) indicates the specific HDAC3 protein band.
-
GC4‐2 cells were treated with 10 ng/ml of IGF‐1 for different periods of time followed by IP and Western blots with the indicated antibodies. The asterisk (*) indicates the specific HDAC3 protein band.
-
H–JC4‐2 cells were transfected with indicated siRNAs and treated with 10 ng/ml of IGF‐1 for 30 min and followed by IP and Western blots with the indicated antibodies. The asterisks (*) indicate the specific HDAC3 protein bands.
-
KA hypothetical model depicting roles of HDAC3 and APPL1 in growth factor (GF)‐induced AKT activation. In the absence of the interaction of GF with a receptor tyrosine kinase (RTK), HDAC3 and APPL1 drift around in the cytosol. As a result, AKT becomes highly acetylated and resistant to be polyubiquitinated. Upon GF stimulation, RTK recruits APPL1, which in turn functions as a scaffold facilitating HDAC3‐mediated deacetylation of AKT, thereby making AKT poised for further activation by polyubiquitination. Activation of this deacetylase‐dependent function of HDAC3 may also require the binding by the deacetylase activating domain of SMRT.
Source data are available online for this figure.
