Figure 2.
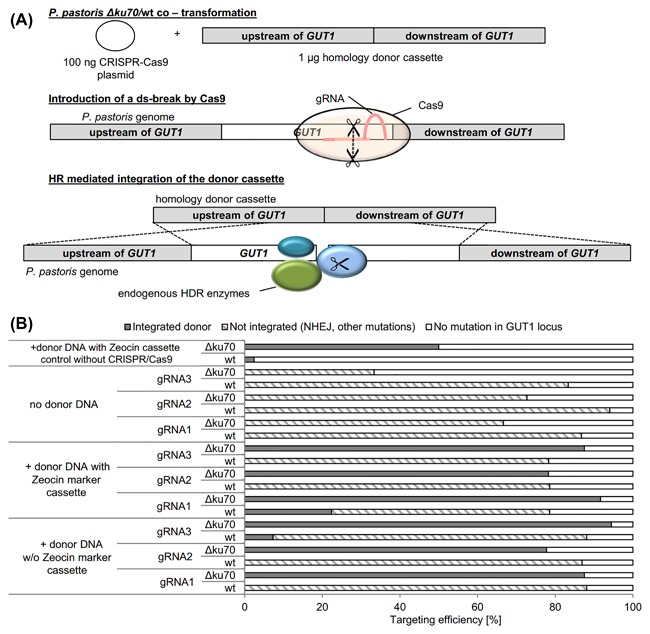
Markerless donor cassette integration with efficiencies approaching 100% in a P. pastoris ku70 deletion strain. A, Principle of CRISPR/Cas9 mediated integration of donor cassettes to allow the complete deletion of genomic DNA. As first step P. pastoris Δku70 was co‐transformed with 100 ng CRISPR/Cas9 plasmid and a linear homologous donor cassette (1 μg). The nuclease Cas9 introduced a double strand break at the target locus, which was the starting point for cellular repair. Host cell HR enzymes mediate the integration of the homologous donor fragment. CRISPR/Cas9 plasmids can be cured by growth on non‐selective media. The elements are not drawn to scale. B, CRISPR/Cas9 mediated integration of homologous donor cassettes does not strongly affect the wildtype strain, but improves specific integration in the P. pastoris Δku70 strain. Competent cells were co‐transformed with 100 ng CRISPR/Cas9 circular plasmid DNA and 1 μg donor DNA to target GUT1. P. pastoris CBS 7435 wildtype results have previously been reported.32 As control solely one μg of the donor DNA was used for the transformation. Upon cultivating random transformants in 96‐DWPs, cell material was transferred with a metallic stamp on minimal media agar plates with either glucose (BMD1) or glycerol (BMG1) as carbon source to determine the targeting efficiency. The integration of the donor cassettes was verified by sequencing of >10 glycerol deficient transformants per CRISPR/Cas9 plasmid—donor DNA combination. Mean values and standard deviations of biological triplicates are shown
