Abstract
Background
At later stages of chronic kidney disease (CKD), a pattern of linear and irreversible renal function decline is thought to be the most common. The objective of this study was to describe the characteristics of the different patterns of CKD progression, and to investigate potentially modifiable factors associated with the rate of decline of renal function.
Methods
This was a retrospective, observational study in a cohort of adult patients with CKD Stage 4 or 5 not on dialysis. Decline in renal function was estimated as the slope of the individual linear regression line of estimated glomerular filtration rate (eGFR) over time. The following patterns of CKD progression were considered: unidentifiable, linear, nonlinear (curvilinear) and positive (improvement of renal function).
Results
The study group consisted of 915 patients (mean ±SD age 65 ± 14 years, 48% females, median follow-up time 16 months). A linear pattern was observed in 38%, unidentifiable in 23%, nonlinear in 24% and positive in 15% of the study patients. The mean eGFR slope was: −3.35 ± 4.45 mL/min/year. Linear and unidentifiable patterns were associated with more rapid loss of renal function. By multiple linear and logistic regression analysis, the magnitude of proteinuria, the systolic blood pressure and the treatment with dual renin–angiotensin system blockade were associated with more rapid CKD progression. On the contrary, older age and discontinuation of commonly prescribed medication with potential influence on renal function or eGFR measurements were associated with slower CKD progression.
Conclusions
A majority of patients with advanced CKD show patterns of renal function decline different from linear, and several of the main determinants of CKD progression are potentially modifiable.
Keywords: chronic kidney disease, dual blockade renin–angiotensin system, patterns of CKD progression, proteinuria
Introduction
Until recent years, it was thought that chronic kidney disease (CKD) exhibited a predominantly linear progression pattern [1–3], with a more rapid decline of renal function at later stages [4–6]. Thus, it seemed difficult to slow or halt the progression of CKD at Stage 4 or 5.
However, recent observational studies have shown that patterns of CKD progression may be highly heterogeneous, and trajectories of glomerular filtration rate (GFR) over time can fit patterns different from linear [7–13]. Knowledge of the determinants of these different CKD progression patterns may be of great interest for the management of this disease.
The aims of this observational study in a cohort of CKD Stages 4 and 5 patients were to describe the main characteristics of the different patterns of progression, analyse the major determinants of the rate of decline of renal function and investigate potentially modifiable factors that could influence on CKD progression.
Materials and methods
This was a longitudinal, retrospective observational study in an incident cohort of adult patients with CKD Stages 4 and 5 not on dialysis, who were admitted to our CKD outpatient clinic between January 2000 and December 2014 due to progressive decline of kidney function.
The inclusion criterion was: having at least three consecutive measurements of estimated GFR (eGFR) in a follow-up period >3 months. Patients with recent acute kidney injury or those with glomerular diseases or vasculitis under immunosuppressive therapy were excluded.
Demographic features, clinical parameters and prescribed medication were obtained from medical records. Davies comorbidity index [14] at the time of the study entry was used to categorize the aggregated diagnosis of comorbid conditions into three subgroups: absence, mild-moderate or severe.
Baseline biochemical parameters included for descriptive purposes were: haemoglobin, serum creatinine, uric acid, calcium, phosphate, bicarbonate, parathyroid hormone, albumin, haemoglobin A1c (only in diabetic patients), high-sensitivity C-reactive protein levels (data available in 675 patients) and 24-h urinary protein excretion (g/g creatinine). All measurements were performed in the same central laboratory of our hospital using standard biochemical methods. The abbreviated Modification of Diet in Renal Disease equation (MDRD) was used to estimate GFR (eGFR) [15].
Patients were followed regularly every 30–90 days. Decline in renal function was estimated as the slope of the individual linear regression line of eGFR over follow-up time, expressed as ±mL/min/1.73 m2/year. Negative or positive values of this parameter indicate renal disease progression or renal function improvement, respectively.
The best-fitting model of eGFR over individual follow-up time was determined in each patient for defining the pattern of CKD progression. The following models were considered: linear, quadratic and cubic. An automatic curve estimation procedure was used, and we assessed model performance by F-value and the coefficient of determination (R2).
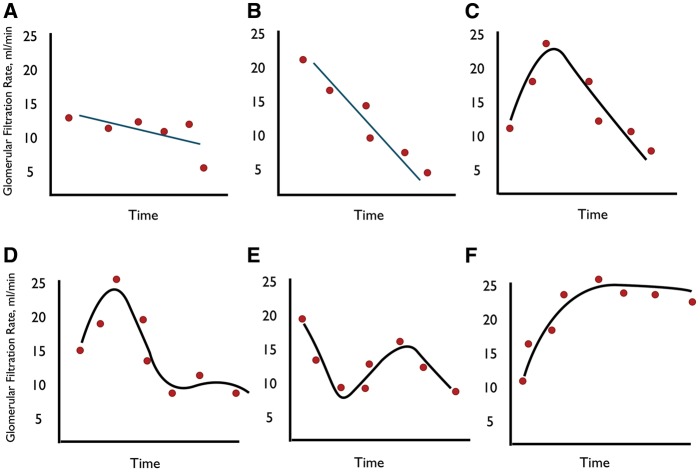
When the relationship between eGFR over time did not fit any statistical significance model (P ≥ 0.05), the pattern of CKD progression was considered as ‘unidentifiable’ (Figure 1A); when the best-fitting model was linear, the pattern of progression was considered as ‘linear’ (Figure 1B); when the best-fitting model was curvilinear (quadratic or cubic), the pattern was considered as ‘nonlinear’ (Figure 1C–E). Finally, all positive slopes were considered as ‘positive pattern’ or renal function improvement (Figure 1F).
Fig. 1.
Example plots illustrating the different patterns of CKD progression. (A) Unidentifiable pattern in which the relationship between eGFR over time does not fit any significant linear or curvilinear pattern. (B) Linear pattern. (C) Nonlinear quadratic pattern. (D) and (E) Nonlinear cubic pattern. (F) Positive pattern with sustained improvement of renal function.
Study design and statistical methods
This was a retrospective observational study in which demographic, clinical and biochemical characteristics of patients were described according to the pattern of CKD progression.
The main determinants of CKD progression were analysed using multiple linear regression models. In addition, multiple logistic regression models were also utilized to analyse associations between covariates and the velocity of CKD progression using a categorical variable. We used the arithmetic mean of eGFR slope of the whole study group as the cutoff value, and considered slow or fast progression if each individual eGFR slope was higher or lower than this value, respectively.
The most common medications used in these patients were included in the description of progression patterns and as covariates in multivariable analyses.
The following medications were included: diuretics, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), dual blockade (concomitant treatment with two of the following drugs: ACEI, ARB or aliskiren), β-blockers, calcium-channel blockers, statins and antiplatelet drugs.
We also included a covariate that we called ‘major treatment modifications’ (MTM), which integrated some modifications of treatment performed at baseline. These changes consisted of discontinuation of vitamin D analogues and/or fibrates and/or allopurinol (this latter medication only in patients with suspected allopurinol hypersensitivity syndrome).
The rates of all-cause hospitalization during the study period were also included in the descriptive analysis, as an indicator of clinical instability.
Other therapeutic measures such as dietary protein restriction, prevention of contrast-induced nephropathy and avoidance of other potential nephrotoxic drugs, treatment with sodium bicarbonate to correct metabolic acidosis, erythropoiesis-stimulating agents to reverse anaemia and measures to control serum phosphate (diet and/or binders) were not included as covariates in the multivariate analysis, although these treatments were indicated to all who required them.
The rest of the covariates included in the multivariate analysis were: age, sex, comorbidity index, current smoking, diabetes mellitus, systolic and diastolic blood pressure measured at the first visit, baseline eGFR and urinary protein excretion.
Due to the long study period, and in an attempt to adjust the hypothetical improvement over time in practice management and treatment guidelines, we also included a variable in which points were allocated to each one of the three consecutive 5-year study periods. Thus, the early period (2000–04) received 1 point, the mid-period (2005–09) 2 points and the late period (2010–14) 3 points.
Cox proportional hazards regression models were used to analyse the main determinants of death before dialysis had been initiated. The proportional hazard assumption was checked graphically (log–log Kaplan–Meier curves) for all covariates.
To estimate the cumulative incidence for requiring dialysis therapy while accounting for the competing risks of dying before dialysis initiation, a competing-risk proportional hazards regression model was built using the method of Fine and Gray, according to Putter et al.'s description [16]. The same covariates selected in the Cox model were also re-analysed with the competing-risk model, and the sub-distribution hazard ratios were estimated.
Parametric and non-parametric tests were chosen as appropriate for descriptive comparisons of continuous variables, and chi-squared test for categorical variables.
Descriptive statistics are presented as mean and standard deviation, or median and interquartile ranges (IQR) for continuous variables, and absolute values and percentages for categorical variables. A P < 0.05 was considered to be significant. All P-values are reported two-sided. Analyses were performed using IBM SPSS Statistics 21.0 (IBM Corp., Armonk, NY, USA), and STATA 11.1 (StataCorp, College Station, TX, USA).
Results
Patients
During the study period, data were retrievedfrom 1383 patients, of whom 944 patients fulfilled the inclusion criteria, and 29 were excluded as they were found to have recent acute kidney injury or were under immunosuppressive therapy for primary glomerulonephrities or vasculitis. Thus, the study group consisted of 915 patients, whose demographic, clinical and biochemical characteristics are detailed in Table 1.
Table 1.
Demographic, clinical characteristics and outcome of the whole study group, and according to CKD progression patterns
| Variable | Total | Unidentifiable | Linear | Nonlinear | Positive | Pa |
|---|---|---|---|---|---|---|
| Patients (%) | 915 | 213 (23) | 349 (38) | 215 (24) | 138 (15) | |
| Age, years (SD) | 65 (14) | 67 (13) | 63 (15) | 64 (14) | 70 (13) | <0.0001 |
| Sex, men (%) | 475 (52) | 118 (55) | 163 (47) | 114 (53) | 80 (58) | 0.073 |
| Body mass index, kg/m2 (SD) | 29.5 (5.9) | 30.2 (6.1) | 29.3 (5.8) | 29.2 (5.9) | 29.6 (5.3) | 0.234 |
| Current smokers | 157 (17) | 33 (16) | 63 (18) | 42 (20) | 19 (14) | 0.461 |
| Comorbidity index (absence/mild-moderate/ severe) | 380 (41)/ 445 (49)/ 90 (10) | 67 (32)/ 118 (55)/ 28 (13) | 169 (48)/ 152 (44)/ 28 (8) | 101 (47)/ 100 (46)/ 14 (7) | 43 (31)/ 75 (54)/ 20 (15) | <0.0001 |
| Diabetes mellitus | 330 (36) | 101 (47) | 112 (32) | 70 (33) | 47 (34) | 0.003 |
| Aetiology CKD | ||||||
| Unknown | 370 (40) | 81 (38) | 124 (35) | 91 (42) | 74 (53) | <0.0001 |
| Glomerulonephrities | 93 (10) | 15 (7) | 43 (12) | 24 (11) | 11 (8) | |
| Diabetic nephropathy | 206 (23) | 66 (31) | 81 (23) | 38 (18) | 21 (15) | |
| Interstitial | 110 (12) | 24 (11) | 40 (12) | 30 (14) | 16 (12) | |
| APKD | 71 (8) | 13 (6) | 41 (12) | 14 (6) | 3 (2) | |
| Ischaemic | 45 (5) | 10 (5) | 13 (4) | 10 (5) | 12 (9) | |
| Others | 20 (2) | 4 (2) | 7 (2) | 8 (4) | 1 (1) | |
| Systolic blood pressure, mmHg | 158 (27) | 162 (28) | 161 (27) | 155 (24) | 147 (25) | <0.0001 |
| Diastolic blood pressure, mmHg | 87 (14) | 87 (14) | 89 (14) | 87 (14) | 81 (14) | <0.0001 |
| Baseline eGFR, mL/min/1.73 m2 | 14.7 (4.5) | 14.0 (4.4) | 15.1 (4.3) | 14.8 (4.8) | 14.6 (4.9) | 0.063 |
| Mean follow-up time, months | 23.2 (21.9) | 13.9 (14.3) | 22.8 (19.6) | 31.0 (25.5) | 26.8 (25.7) | <0.0001 |
| Number of samples (median, IQR) | 7 (5–11) | 5 (4–7) | 8 (5–12) | 10 (7–14) | 6 (4–10) | <0.0001 |
| eGFR slope, mL/min/1.73 m2/year | −3.35 (4.44) | −4.07 (4.41) | −5.26 (3.96) | −3.19 (2.62) | +2.36 (2.87) | <0.0001 |
| Hospitalization rate, days/year | 3.1 (6.7) | 6.3 (9.1) | 1.6 (4.5) | 1.6 (3.8) | 4.1 (8.2) | <0.0001 |
| Dialysis initiation | 583 (64) | 125 (59) | 271 (78) | 162 (75) | 25 (18) | <0.0001 |
| Death before dialysis initiation | 142 (16) | 41 (19) | 37 (11) | 30 (14) | 35 (25) | <0.0001 |
| Lost to follow-up | 14 (2) | 3 (1) | 0 (0) | 2 (1) | 9 (7) | <0.0001 |
ANOVA or chi-square inter-groups.
CKD progression
The mean eGFR slope in the overall sample was: −3.35 ± 4.44 mL/min/1.73 m2/year. This parameter followed a Gaussian distribution (Figure 2) with a minimum and maximum values of −25.5 and +15.5 mL/min/1.73 m2/year, respectively.
Fig. 2.

Histogram representing frequency distribution of eGFR slopes in the whole study group.
Mean eGFR slopes were consistently improving over the study periods (early, mid and late): −3.81 ± 4.03 versus −3.35 ± 4.28 versus −2.74 ± 5.08 mL/min/year, respectively [P = 0.016, analysis of variance (ANOVA)].
Patterns of progression of CKD
In all, 349 patients (38%) showed a linear pattern of CKD progression. In 213 patients (23%), the regression between eGFR over time did not fit any statistical significant pattern (unidentifiable pattern). In 215 patients (24%), the best-fitted model was nonlinear (curvilinear), while the remaining 138 patients (15%) showed improvement of renal function (positive pattern).
The nonlinear progression pattern consisted of 100 patients with quadratic and 115 with cubic curves.
Main characteristics of patients according to CKD progression patterns
Tables 1 and 2 show the main characteristics of patients according to CKD progression patterns.
Table 2.
Baseline biochemical characteristics and main treatments of the whole study group, and according to CKD progression patterns
| Variable | Total | Unidentifiable | Linear | Nonlinear | Positive | Pa |
|---|---|---|---|---|---|---|
| Baseline biochemical parameters | ||||||
| Haemoglobin, g/dL | 11.4 (4.3) | 11.7 (8.4) | 11.3 (1.7) | 11.5 (1.4) | 11.5 (1.9) | 0.696 |
| Serum uric acid, mg/dL | 7.6 (1.9) | 7.6 (1.9) | 7.3 (1.9) | 7.9 (1.9) | 7.7 (2.3) | 0.015 |
| Serum total calcium, mg/dL | 9.2 (0.8) | 9.1 (0.9) | 9.2 (0.7) | 9.4 (0.8) | 9.3 (0.7) | <0.0001 |
| Serum phosphate, mg/dL | 4.7 (1.0) | 4.8 (1.1) | 4.6 (0.9) | 4.8 (1.1) | 4.4 (1.1) | 0.014 |
| Serum bicarbonate, mmol/L | 21.4 (4.0) | 21.2 (4.3) | 21.2 (3.8) | 21.8 (3.9) | 22.3 (4.3) | 0.031 |
| Haemoglobin A1c in diabetic patients, % | 6.73 (1.42) | 6.72 (1.32) | 6.77 (1.47) | 6.92 (1.56) | 6.39 (1.28) | 0.260 |
| Serum albumin, g/dL | 3.9 (0.4) | 3.9 (0.4) | 3.9 (0.4) | 3.9 (0.5) | 4.0 (0.4) | 0.240 |
| Serum C-reactive protein, mg/Lb | 3.53 (1.16–9.20) | 4.04 (1.65–11.50) | 3.22 (1.02–8.96) | 2.83 (0.87–6.87) | 4.86 (1.40–13.51) | 0.011 |
| Urinary protein excretion, g/g creatinine | 2.13 (2.35) | 2.65 (2.76) | 2.31 (2.16) | 1.96 (2.45) | 1.13 (1.52) | <0.0001 |
| Treatments | ||||||
| Successful arterial-venous fistulae | 283 (31) | 61 (29) | 137 (39) | 67 (32) | 18 (13) | <0.0001 |
| Diuretics | 595 (65) | 142 (67) | 209 (60) | 155 (72) | 89 (65) | 0.029 |
| ACEI or ARB | 693 (76) | 170 (80) | 275 (79) | 157 (73) | 91 (66) | 0.008 |
| Dual RAS blockade (ACEI + ARB) | 79 (9) | 18 (9) | 40 (12) | 17 (8) | 4 (3) | 0.020 |
| β-blockers | 208 (23) | 56 (26) | 73 (21) | 45 (21) | 34 (25) | 0.412 |
| Calcium-channel blockers | 441 (48) | 104 (49) | 175 (50) | 101 (47) | 61 (44) | 0.665 |
| Antiplatelet drugs | 305 (33) | 81 (38) | 102 (29) | 38 (32) | 54 (39) | 0.068 |
| Statins | 438 (48) | 111 (52) | 171 (49) | 94 (44) | 62 (45) | 0.299 |
| MTMc | 93 (10) | 28 (13) | 25 (7) | 14 (7) | 26 (19) | <0.0001 |
ANOVA or chi-square inter-groups.
Data available in 675 patients, expressed as median and interquartile ranges, and compared by Kruskal–Wallis test.
MTM: discontinuation of vitamin D analogues, fibrates and/or allopurinol (only in patients with suspected allopurinol hypersensitivity) at baseline.
Of note, patients with the unidentifiable pattern had the shortest follow-up time and the lowest number of kidney function measurements (overall median of five determinations). Patients with unidentifiable and positive patterns were older, and exhibited greater numbers of comorbid conditions and hospitalization rates.
The slope of CKD progression was steeper (faster progression) in unidentifiable and linear patterns, as opposed to the group with the nonlinear pattern. Consistent with this finding, both mean systolic and diastolic blood pressure, and urinary protein excretion were higher in the unidentifiable and linear patterns than in the nonlinear pattern.
Dual blockade of the renin–angiotensin system (RAS) was prescribed more frequently in patients with unidentifiable or linear CKD progression patterns. The mean eGFR slope of the 79 patients on dual RAS blockade was significantly steeper than that of the rest of the study patients (−6.06 ± 5.36 versus −3.09 ± 4.27 mL/min/1.73 m2/year, P < 0.0001).
MTM were more frequently performed among patients with renal function improvement (19%). The mean eGFR slope in the 93 patients in whom one or more drugs suspected to be involved in the decline of kidney function were discontinued, was more favourable than that of the rest of the study patients (−2.06 ± 3.98 vs −3.49 ± 4.47 mL/min/1.73 m2/year, P = 0.003). More specifically, the mean eGFR slope of the 58 patients who discontinued vitamin D analogues was: −2.26 ± 3.69 mL/min/1.73 m2/year; 30 patients who discontinued fibrates: −1.82 ± 3.93 mL/min/1.73 m2/year; and 9 patients in whom allopurinol was withdrawn: −1.61 ± 5.30 mL/min/1.73 m2/year.
The aetiologies of CKD according to the pattern of progression are detailed in Table 1. No significant differences were found in the frequency distribution of aetiologies, although it is worth mentioning the low percentage of diabetic nephropathy among patients with patterns associated with slower progression (nonlinear and positive), and the predominance of unknown aetiology and atherosclerotic vascular disease among patients with the positive pattern.
In diabetic patients, levels of haemoglobin A1c did not significantly differ across the four different patterns of CKD progression (Table 2).
Median C-reactive protein levels were higher in patients with unidentifiable and positive patterns (Table 2), consistent with their respective comorbidity burden.
Factors associated with faster CKD progression
By multiple linear regression analysis, the best determinants of eGFR slope are shown in Table 3. Covariates associated with slower progression were: age (slower progression in ageing patients), and discontinuation of drugs suspected to be involved in kidney function decline. On the contrary, higher systolic blood pressure, the magnitude of urinary protein excretion and the treatment with dual RAS blockade were associated with faster progression.
Table 3.
Multiple linear regression model for eGFR slope (mL/min/1.73 m2/year)
| Variable | B coefficient | 95% CI B coefficient | β | P |
|---|---|---|---|---|
| Age, years | 0.045 | 0.026; 0.064 | 0.142 | <0.0001 |
| Sex, male = 1 | −0.615 | −1.136; −0.094 | −0.069 | 0.021 |
| Study periods; early = 1, mid = 2, late = 3 | 0.368 | 0.034; 0.701 | 0.066 | 0.031 |
| Systolic blood pressure, × cmHg | −0.225 | −0.325; −0.125 | −0.137 | <0.0001 |
| Proteinuria, g/g creatinine | −0.630 | −0.745; −0.514 | −0.333 | <0.0001 |
| Dual RAS blockade (0, 1) | −1.475 | −2.424; −0.526 | −0.093 | 0.002 |
| MTMa (0, 1) | 1.290 | 0.420; 2.161 | 0.088 | 0.004 |
| Constant | −1.794 | −3.723; 0.136 |
Not in equation: comorbidity index, diabetes mellitus, baseline eGFR, smokers, diastolic blood pressure, diuretics, ACEI or ARB treatment, β-blockers, calcium-channel blockers, antiplatelet drugs, statins.
MTM: discontinuation at baseline of vitamin D analogues, fibrates and/or allopurinol (only in patients with suspected allopurinol hypersensitivity).
These same covariates determined the best predictive equation for faster progression of CKD defined as eGFR slope lower than the mean value of the whole study group (Table 4).
Table 4.
Multiple logistic regression model for faster CKD progression
| Variable | Odds ratio | 95% CI odds ratio | P |
|---|---|---|---|
| Age, years | 0.980 | 0.970; 0.990 | <0.0001 |
| Sex, male = 1 | 1.401 | 1.045; 1.879 | 0.024 |
| Systolic blood pressure, ×10 mmHg | 1.081 | 1.021; 1.144 | 0.008 |
| Proteinuria, g/g creatinine | 1.413 | 1.301; 1.534 | <0.0001 |
| Treatment with dual RAS blockade (0, 1) | 2.163 | 1.268; 3.689 | 0.005 |
| MTMa (0, 1) | 0.445 | 0.259; 0.764 | 0.003 |
Not in equation: study periods, smoking, diastolic blood pressure, baseline eGFR, diabetes, comorbidity index, diuretics, ACEI or ARB therapy, β-blockers, calcium-channel blockers, antiplatelet drugs, statins.
MTM: discontinuation at baseline of vitamin D analogues, fibrates and/or allopurinol (only in patients with suspected allopurinol hypersensitivity).
In diabetic patients, mean levels of haemoglobin A1c were almost identical in fast and slow progressors (6.73 ± 1.32% versus 6.73 ± 1.49%, respectively). Haemoglobin A1c levels did not correlate with eGFR slopes (β = 0.012; P = 0.830).
Median C-reactive protein levels were similar in slow and in fast progressors (3.65 versus 3.48 mg/L, P = 0.840 Mann–Whitney test). C-reactive protein levels correlated weakly but significantly with eGFR slopes in a univariate model (β = 0.085; P = 0.027). However, C-reactive protein levels no longer remained significantly associated with eGFR slopes when demographic characteristics were included in a multivariate model (β = 0.072; P = 0.061).
Determinants of worse outcomes (death or need for dialysis)
During a median follow-up period of 16 months (IQR: 8–30 months), 583 patients initiated dialysis (64%), and 142 patients died before dialysis had been initiated (16%).
By Cox proportional hazards regression model, the main determinants of mortality before dialysis initiation, and of dialysis initiation adjusted for competing risk of death, are shown in Table 5.
Table 5.
Cox proportional hazard regression model for association between covariates and mortality before dialysis initiation, or dialysis initiation adjusted for competing risk of death (sub-distribution hazards model of Fine and Gray)
| Variable | Mortality |
P | Dialysis initiation |
P | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI hazard ratio | Sub-hazard ratio | 95% CI sub-hazard ratio | |||
| Age, years | 1.096 | 1.071; 1.123 | <0.0001 | 0.978 | 0.971; 0.985 | <0.0001 |
| Sex, male = 1 | 1.342 | 0.914; 1.968 | 0.133 | 1.397 | 1.161; 1.686 | <0.0001 |
| Comorbidity index (0, 1, 2) | 1.955 | 1.425; 2.681 | <0.0001 | 0.805 | 0.651; 0.995 | 0.045 |
| Study periods, Early = 1; Middle = 2; Recent = 3 | 1.005 | 0.761; 1.325 | 0.972 | 0.892 | 0.783; 1.014 | 0.082 |
| Current smokers (0, 1) | 2.485 | 1.514; 4.078 | <0.0001 | 0.862 | 0.676; 1.098 | 0.228 |
| Systolic blood pressure, ×10 mmHg | 1.045 | 0.972; 1.124 | 0.235 | 1.058 | 1.020; 1.098 | 0.002 |
| Baseline eGFR, ml/min/1.73 m2 | 0.951 | 0.913; 0.990 | 0.015 | 0.877 | 0.856; 0.899 | <0.0001 |
| Proteinuria, g/g creatinine | 1.254 | 1.174; 1.339 | <0.0001 | 1.105 | 1.053; 1.159 | <0.0001 |
| Diabetes mellitus (0, 1) | 1.209 | 0.812; 1,799 | 0.350 | 1.323 | 1.030; 1.700 | 0.028 |
| Diuretics (0, 1) | 1.408 | 0.952; 2.082 | 0.086 | 1.051 | 0.872; 1.265 | 0.602 |
| ACEI or ARB treatment (0, 1) | 0.574 | 0.389; 0.847 | 0.005 | 1.002 | 0.788; 1.273 | 0.989 |
| Dual blockade RAS (0,1) | 0.601 | 0.236; 1.532 | 0.286 | 1.333 | 1.005; 1.768 | 0.046 |
| β-blockers (0, 1) | 1.113 | 0.727; 1.703 | 0.622 | 1.291 | 1.038; 1.606 | 0.022 |
| Calcium-channel blockers (0, 1) | 0.705 | 0.486; 1.024 | 0.066 | 1.162 | 0.970; 1.392 | 0.103 |
| Antiplatelets (0, 1) | 1.086 | 0.764; 1.544 | 0.645 | 0.852 | 0.691; 1.052 | 0.136 |
| Statins (0, 1) | 0.861 | 0.595; 1.246 | 0.427 | 1.118 | 0.929; 1.346 | 0.238 |
| MTM (0, 1) | 0.134 | 0.018; 0.966 | 0.046 | 0.974 | 0.748; 1.268 | 0.846 |
Summarizing these results, patients who died before dialysis had been initiated were characterized by the following clinical profile: ageing patient with high degree of comorbidity, current smoker, with proteinuric renal disease, but treated less frequently with ACEI or ARB. On the other hand, the most notable characteristics of patients who needed earlier initiation of dialysis were as follows: younger male with lower baseline renal function, higher systolic blood pressure and proteinuric renal disease (more common diabetic), treated more frequently with dual blockaded of RAS and/or β-blockers.
Figure 3 shows ‘survival without dialysis’ curves according to the pattern of CKD progression, and Figure 4 shows ‘survival without dialysis’ curves according to the rate of CKD progression (faster or slower).
Fig. 3.
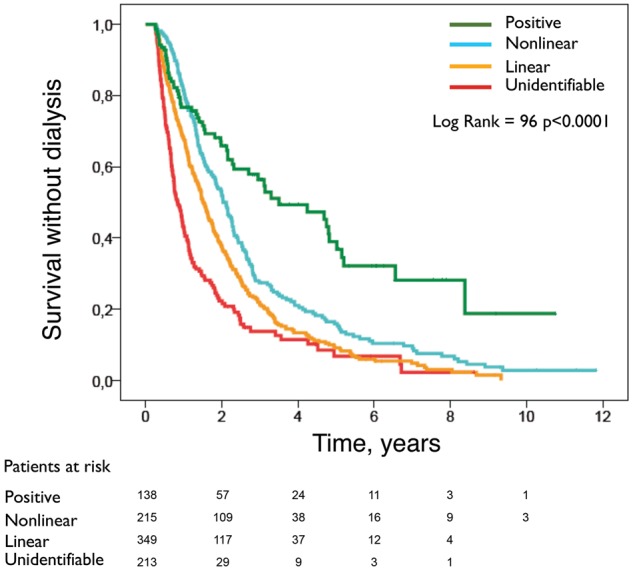
Kaplan–Meier ‘survival without dialysis’ curves according to different patterns of progression.
Fig. 4.
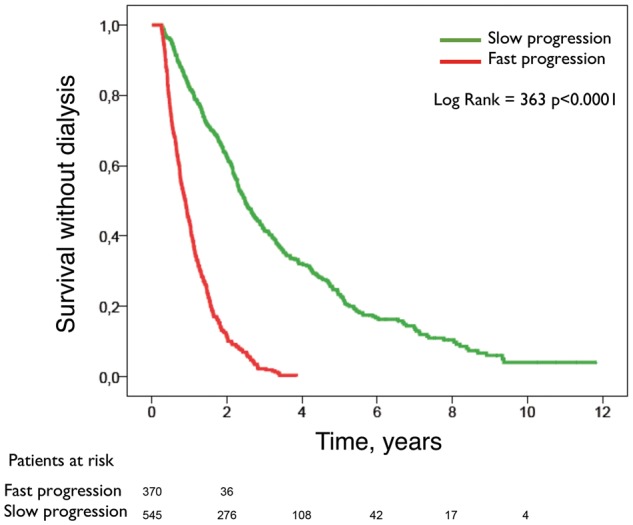
Kaplan–Meier ‘survival without dialysis’ curves according to the rate of progression of CKD (faster or slower).
Discussion
In this observational study, four different patterns of CKD progression could be distinguished: unidentifiable, linear, nonlinear and positive.
The unidentifiable pattern, in which the relationship between eGFR over time did not fit any predictable pattern, accounted for 23% of the study patients. These patients were older, had greater comorbidity, especially diabetes mellitus, higher systolic blood pressure and urinary protein excretion, and they had the worst outcomes (faster CKD progression and lesser survival rate with no dialysis).
A more frequent general frailty and clinical instability in these patients, as reflected by their greater comorbidity index and hospitalization rate, may explain this unpredictable pattern of CKD progression. These patients usually present abrupt changes in their clinical course and kidney function, mainly related to cardiovascular (congestive heart failure) and infectious complications that result in hospitalization, and they eventually need the initiation of unplanned dialysis. The shorter follow-up time in this group could also influence the lack of a clearly defined pattern of CKD progression.
Although the linear pattern of CKD progression was the most common, it accounted for only 38% of the study patients. Some characteristics of these patients, such as higher systolic blood pressure and urinary protein excretion, matched those exhibited by patients with the unidentifiable pattern. However, patients with the linear pattern were younger and had fewer comorbidities than those with the unidentifiable pattern. In addition, primary glomerulonephrities and adult polycystic kidney disease (APKD) were the predominant CKD aetiologies in this group.
The linear pattern was also associated with faster decline of kidney function and earlier need for dialysis initiation. Among the potential factors involved, dual RAS blockade treatment was more commonly prescribed in these patients, and although some concerns about confounding by indication should be considered, dual RAS blockade treatment was significant and independently associated with faster CKD progression in adjusted multivariate analyses.
Associations between dual RAS blockade treatment and faster CKD progression have already been described in other studies, in patients both with and without diabetic nephropathy [17–19], and although these results should be interpreted cautiously, it would be advisable to take them into consideration when this therapy is indicated to patients with advanced CKD.
The nonlinear pattern of CKD progression was also common —24% of patients. Despite the scant differences in clinical characteristics of patients with linear and nonlinear patterns, those with the nonlinear pattern showed a slower decline of renal function. The unsteady CKD progression in the nonlinear pattern may be explained by transient or partial response to the numerous therapeutic interventions performed to these patients, which may eventually help to delay the need for dialysis.
The positive pattern or renal function improvement was observed in 15% of the study patients. These patients were older and had higher degrees of comorbidity; however, mean blood pressure and proteinuria were the lowest compared with those of the rest of the groups. Interestingly, these patients were being treated less frequently with ACEI or ARB or dual RAS blockade, and in 19% of them MTM were performed at baseline.
Vitamin D analogues, fibrates and allopurinol are drugs commonly prescribed in CKD patients that can be associated with real [20–23] or apparent decrease of renal function due to misinterpretations of eGFR from equations based on serum creatinine measurements [24, 25]. Well aware of this potential adverse effect, we decided long ago to discontinue these drugs in a systematic manner to all patients admitted to our CKD clinic (allopurinol was only stopped in cases in which hypersensitivity to this medication was suspected). This intervention, which we called MTM, was performed in ∼10% of the study patients, and this covariate resulted to be independently associated with a more favourable CKD progression in multivariable analyses.
During the followed-up period, almost two-thirds of the study patients needed dialysis, 16% died before dialysis had been initiated and 20% had a favourable outcome—survival without dialysis or kidney transplantation.
As would be expected, baseline kidney function and factors associated with more rapid CKD progression (higher blood pressure and urinary protein excretion) were the best determinants of the need for dialysis adjusted for the competing risk of dying before dialysis initiation. While the treatment with either ACEI or ARB seemed to be associated with less mortality, the concomitant prescription of both drugs (dual blockade of RAS) or the use of β-blockers was significantly associated with earlier need of dialysis.
In this study, a high proportion of elderly patients showed a slow CKD progression. Nearly two-thirds (64%) of the study patients over 65 years of age showed less steep eGFR slopes (greater than −3.35 mL/min/1.73 m2/year). This finding has already been described in previous studies [26–28], although the causal link between ageing and slow CKD progression remains elusive. One explanation widely supported is that ‘ageing kidney’ and its associated outcome characteristics [29] could be the predominant aetiology of renal function impairment in a high proportion of elderly CKD patients. Besides, other clinical characteristics and confounders related with ageing, such as better compliance with prescribed medication and dietary advice (authors’ subjective perception), fewer current smokers and inaccuracy of creatinine-based estimations of GFR, may also influence the rate of renal function decline in our elderly CKD population.
One important conclusion that can be drawn from these results is that renal function decline at later stages of CKD is not uniformly linear, and the velocity of the progression can vary widely. Thus, the characteristics associated with these different patterns of CKD progression should be considered in clinical studies and trials recruitment, and from a clinical practice standpoint, for proper planning and scheduling of the initiation of dialysis or pre-emptive kidney transplantation.
Potential limitations of this study need to be acknowledged. Due to the observational and retrospective nature of the study no causal relationships could be established. The study was single centre, and the overall outcomes and kidney function could be highly influenced by the treatment protocols of our clinic, thus limiting the reproducibility of some of the results from this study.
Renal function was not measured by gold standard methods. The precision and accuracy of creatinine-based formulas for estimating the renal function can be affected by numerous factors, which may eventually influence the estimation of CKD progression observed in this study.
In conclusion, this study shows that, in addition to highly expected risk factors for CKD progression (age, sex, arterial hypertension, proteinuria), other potential modifiable factors, mainly related to the adverse effects of commonly prescribed medication, may influence significantly the rate of renal function decline of CKD patients at later stages. Interactions among these factors result in different patterns of progression, the identification of which may be useful for optimizing the care of patients with advanced CKD.
Conflict of interest statement
None declared.
References
- 1. Mitch WE, Walser M, Buffington GA. et al. A simple method of estimating progression of chronic renal failure. Lancet 1976; 2: 1326–1328 [DOI] [PubMed] [Google Scholar]
- 2. Levey A, Perrone R, Madias N.. Serum creatinine and renal function. Ann Rev Med 1988; 39: 465–490 [DOI] [PubMed] [Google Scholar]
- 3. Hunsicker LG, Adler S, Caggiula A. et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997; 51: 1908–1919 [DOI] [PubMed] [Google Scholar]
- 4. Lee P, Johansen K, Hsu CY.. End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol 2011; 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhong Y, Muñoz A, Schwartz GJ. et al. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol 2014; 25: 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu RK, Chai B, Roy JA. et al. Abrupt decline in kidney function before initiating hemodialysis and all-cause mortality: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2016; 68: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemley KV, Boothroyd DB, Blouch KL. et al. Modelling GFR trajectories in diabetic nephropathy. Am J Physiol Renal Physiol 2005; 289: F863–F870 [DOI] [PubMed] [Google Scholar]
- 8. O'Hare AM, Batten A, Burrows NR. et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 2012; 59: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Astor BC, Lewis J. et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012; 59: 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Chang A, Rostand SG. et al. A within-patient analysis for time-varying risk factors of CKD progression. J Am Soc Nephrol 2014; 25: 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leffondre K, Boucquemont J, Tripepi G. et al. Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol Dial Transplant 2015; 30: 1237–1243 [DOI] [PubMed] [Google Scholar]
- 12. Xie Y, Bowe B, Xian H. et al. Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am J Kidney Dis 2016; 68: 219–228 [DOI] [PubMed] [Google Scholar]
- 13. Collister D, Ferguson T, Komenda P. et al. The patterns, risk factors, and prediction of progression in chronic kidney disease: a narrative review. Semin Nephrol 2016; 36: 273–282 [DOI] [PubMed] [Google Scholar]
- 14. Davies SJ, Russell L, Bryan J. et al. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis 1995; 26: 353–361 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 16. Putter H, Fiocco M, Geskus RB.. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007; 26: 2389–2430 [DOI] [PubMed] [Google Scholar]
- 17. Frimodt-Møller M, Høj Nielsen A, Strandgaard S. et al. Feasibility of combined treatment with enalapril and candesartan in advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25: 842–847 [DOI] [PubMed] [Google Scholar]
- 18. Mann JF, Tobe S, Teo KK. et al. Is therapy of people with chronic kidney disease ONTARGET? Nephrol Dial Transplant 2010; 25: 42–44 [DOI] [PubMed] [Google Scholar]
- 19. Chan KE, Ikizler TA, Gamboa JL. et al. Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int 2011; 80: 978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christiansen C, Rødbro P, Christensen MS. et al. Deterioration of renal function during treatment of chronic renal failure with 1,25-dihydroxycholecalciferol. Lancet 1978; 2: 700–703 [DOI] [PubMed] [Google Scholar]
- 21. de Zeeuw D, Agarwal R, Amdahl M. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376: 1543–1551 [DOI] [PubMed] [Google Scholar]
- 22. Jun M, Zhu B, Tonelli M. et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 2012; 60: 2061–2071 [DOI] [PubMed] [Google Scholar]
- 23. Stamp LK, Day RO, Yun J.. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol 2016; 12: 235–242 [DOI] [PubMed] [Google Scholar]
- 24. Bertoli M, Luisetto G, Ruffatti A. et al. Renal function during calcitriol therapy in chronic renal failure. Clin Nephrol 1990; 33: 98–102 [PubMed] [Google Scholar]
- 25. Agarwal R, Hynson JE, Hecht TJ. et al. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 2011; 80: 1073–1079 [DOI] [PubMed] [Google Scholar]
- 26. O'Hare AM, Choi AI, Bertenthal D. et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758–2765 [DOI] [PubMed] [Google Scholar]
- 27. De Nicola L, Minutolo R, Chiodini P. et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int 2012; 82: 482–488 [DOI] [PubMed] [Google Scholar]
- 28. Tsai CW, Ting IW, Yeh HC. et al. Longitudinal change in estimated GFR among CKD patients: A 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS One 2017; 12: e0173843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Sullivan ED, Hughes J, Ferenbach DA.. Renal ageing: causes and consequences. J Am Soc Nephrol 2017; 28: 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]