Screening Arabidopsis mutants for sensitivity to low-Mn conditions identifies novel alleles for NRAMP1, a previously known Mn transporter, and PGSIP6, a phosphorylceramide glucuronosyltransferase.
Keywords: Arabidopsis thaliana, Golgi, manganese deficiency, NRAMP1, PGSIP6, screening
Abstract
Manganese (Mn) is an essential micronutrient; however, few genes required for growth under low-Mn conditions have been identified. In this study, we isolated Arabidopsis thaliana mutants sensitive to low-Mn conditions from ethyl methanesulfonate-mutagenized seeds. Among them, we identified the causal genes of two mutants. One mutant (35-34) exhibited a short root phenotype and low Mn concentration in the shoots. The other mutant (30-11) exhibited a small shoot phenotype with Mn concentrations similar to the control. Genetic mapping, allelism tests, and gene complementation tests identified the causal genes as At1g80830 (NRAMP1) for 35-34 and At5g18480 (PGSIP6) for 30-11. NRAMP1 was previously reported to be essential for Mn uptake under low-Mn conditions, thus validating our screening method. PGSIP6 encodes inositol phosphorylceramide glucuronosyltransferase, which is involved in glycosyl inositol phosphorylceramide sphingolipid glycosylation. PGSIP6-green fluorescent protein was localized to the Golgi apparatus, which is consistent with its function in the glycosylation of sphingolipids. Our screening identified a novel gene required for low-Mn tolerance, and we also provide new insights towards understanding the physiological function of PGSIP6.
Introduction
Manganese (Mn) is an essential micronutrient for all known organisms, and is the second most abundant transition element constituting the Earth’s crust (Yang et al., 2008). The dominant form of Mn in aerated soils is the tetravalent state, but only the form Mn (Mn2+) is the available for uptake by plants. Therefore, although soil contains a large amount of Mn, the quantity that can be used by organisms is quite low (Kabata-Pendias, 2011).
Mn deficiency a physiological disorder that is widely observed in agriculture. Mn deficiency often occurs in alkaline or calcareous soils due to the low bioavailability and immobilization of Mn2+ (Marschner, 1995). Typical Mn-deficiency symptoms include diffused interveinal chlorosis in younger leaves due to the low phloem mobility of Mn2+ from old to young leaves (Marschner, 1995; Schmidt et al., 2016). In addition, it has been reported that some species become more susceptible to cold stress and pathogen infection under Mn deficiency (Marschner, 1995). Therefore, it is important to have a better understanding of the adaptation mechanisms to low-Mn conditions.
Mn acting as a component of manganese superoxide dismutase (MnSOD), an important antioxidant defense in mitochondria, exists in nearly all living cells exposed to oxygen (Socha and Guerinot, 2014). Mn is also required as a catalyst in the oxygen-evolving complex (OEC) of photosystem II, which is responsible for water photolysis using sunlight and is present in the thylakoids of plants, algae, and cyanobacteria (Kern et al., 2005; Umena et al., 2011; Schmidt et al., 2016). Mn functions as a co-factor or activator for a wide variety of catalysts in decarboxylation, oxidation–reduction, and hydrolytic reactions (Marschner, 1995).
Mn is also required for glycosylation. ß1,3-galactosyltransferase1 (GALT1) catalyses the transfer of ß1,3-linked galactose residues onto N-glycans (Strasser et al., 2007). α1,3-fucosyltransferases catalyses the addition of L-fucose from GDP-Fuc to the reducing terminal N-acetylglucosamine (GlcNAc) residue of N-glycans (Both et al., 2011). GlcA substitution of xylen 1 (GUX1) catalyses the addition of glucuronosyl substitutions on the xylan backbone (Rennie et al., 2012). All of these enzymes are localized to the Golgi apparatus and require Mn2+ for their activity.
Because Mn plays such an important role in physiological activities, plants are capable of maintaining their cellular Mn concentration within a certain range. Several genes have been reported to regulate Mn distribution in plants. The natural resistance-associated macrophage protein (NRAMP) family are transporters responsible for Mn distribution and exist in a wide variety of species, ranging from bacteria to humans (Cellier et al., 1995; Nevo and Nelson, 2006). There are six NRAMP family members in Arabidopsis thaliana. AtNRAMP1 is localized to the plasma membrane as well as to intracellular membranes including the Golgi apparatus (Cailliatte et al., 2010; Agorio et al., 2017). NRAMP1 is involved in Mn absorption from the soil and is required for the growth under low-Mn conditions (Cailliatte et al., 2010). AtNRAMP2 is localized on the trans-Golgi network and is required for root growth under low-Mn conditions (Gao et al., 2018). AtNRAMP3 and AtNRAMP4 are localized to the vacuolar membrane and have important roles in Mn allocation to the chloroplasts (Lanquar et al., 2010). A double-mutant of AtNRAMP3 and AtNRAMP4 shows impaired growth due to the reduced function of photosystem II under low-Mn conditions (Lanquar et al., 2010). In rice, OsNRAMP5 localized in the plasma membrane contributes to Mn uptake (Ishimaru et al., 2012).
In addition to the NRAMP family, several other Mn transporters have been identified in plants. AtECA3 belongs to the Ca2+-ATPase subfamily, which has also been reported to be involved in Mn transport. It has been shown that AtECA3 is localized to the Golgi apparatus and mutants where its function is disrupted show poor growth under low-Mn conditions (Mills et al., 2008). Iron-regulated transporter 1 (IRT1) is a metal transporter that acts on both Fe and Mn (Vert et al., 2002). Transcripts of HvIRT1 in barley are induced under low-Mn and low-Fe conditions. Since Mn2+ absorption increases in proportion to the expression of HvIRT1, it is thought that it promotes efficient absorption of Mn (Pedas et al., 2008). Arabidopsis metal tolerance protein 11 (AtMTP11), belonging to the cation diffusion facilitator (CDF) family, is localized to pre-vacuolar compartments and confers resistance to excess Mn (Delhaize et al., 2007).
In this study, we isolated mutants sensitive to low-Mn conditions and identified the causal genes. One was a previously known gene, NRAMP1, and the other was PGSIP6, which encodes an enzyme required for the formation of glycosyl inositol phosphorylceramide (GIPC) sphingolipids (Tartaglio et al., 2017). Our study provides new insights connecting GIPC sphingolipid synthesis with a mechanism for low-Mn tolerance in plants.
Material and methods
Plant material and cultivation conditions
Arabidopsis thaliana (L.) Heynh was used as the plant material with Columbia-0 (Col-0) used as the control. Ethyl methanesulfonate (EMS)-mutagenized seeds were obtained from Lehle Seeds (http://www.arabidopsis.com/). T-DNA insertion mutants were obtained from the Arabidopsis BioResource Center (ABRC; http://abrc.osu.edu/) or the Nottingham Arabidopsis Stock Centre (NASC; http://arabidopsis.info/).
Plants were grown on MGRL medium (Fujiwara et al., 1992). To adjust the Mn concentration in the medium, Mn hexahydrate (MnSO4.6H2O) was supplied.
Plant cultivation was conducted as follows. Seeds were soaked in a 10% sodium hypochlorite solution for 5 min and washed five times with sterilized ultrapure water. The sterilized seeds were sown in the MGRL medium and subjected to a vernalization treatment at 4 °C for 1–3 d. The plates were placed in a growth chamber (LPH-350S, Nippon Medical Equipment Co., Ltd.) under long-day conditions (16/8 h light/dark cycle) at 22 °C. After 2 weeks, plants were harvested for analysis.
Screening for mutants sensitive to low-Mn conditions
First, about 15 000 EMS-mutagenized M2 seeds derived from 15 000 M1 seeds were sown onto MGRL medium solidified with 1.2% agar (Type A; Sigma-Aldrich) without Mn supplementation (0 μM Mn). The 0 µM Mn agar plate was calculated to contain 0.47 µM Mn according to Jain et al. (2009). The seeds were grown for 10 d (25 seeds per 140 cm2) and plants with poor growth compared to the wild-type were selected and transplanted to normal Mn conditions (10 μM Mn). At 7 d after transplanting, plants whose growth recovered were selected as mutants. Secondary screening was performed using M3 seeds derived from M2. The seeds were sown in the low- and normal-Mn media (8 seeds per 140 cm2) and after 10–14 d the mutants that showed growth inhibition only in low-Mn conditions were selected as low-Mn-sensitive mutants.
Determination of Mn concentrations
Plants were grown for 14 d under different Mn concentrations. The shoots and roots of 5–10 plants were harvested and pooled for each sample, rinsed twice with ultrapure water, and then dried at 60 °C for 48 h. The weights of the dried samples were measured with an electronic balance, and samples were then transferred to Teflon tubes for nitric-acid digestion. Nitric acid (1 ml) was added to each sample (dry weight 1–5 mg) and digestion took place at 100 °C. After digestion, the samples were dissolved in 2 ml of 0.08 N nitric acid containing 1 ppb indium as an internal standard. Element concentrations were then measured using inductively coupled plasma mass spectrometry (ICP-MS: model SPQ 9700; SII Nano Technology, Seiko).
Map-based cloning
For genetic mapping, mutants 35-34 and 30-11 were crossed with the Landsberg erecta (Ler) accession, and the respective self-fertilized F2 populations were used for mapping. The F2 plants were grown in media without Mn, after which genomic DNA was prepared from those plants showing the mutant phenotype. Simple sequence length polymorphism markers were used for the mapping.
Genome sequencing of mutants by SOLiDTM next-generation sequencing
For mutant 30-11, after rough mapping, a next-generation sequencer system (SOLiD™, Applied Biosystems) was used to identify the mutation in the mapped region, as described previously (Tabata et al., 2013). Genomic DNA extraction was conducted using DNeasy (Qiagen, MD, USA), and sample sequence analysis was performed using the SOLiD system at the University of Tokyo’s Graduate School of Frontier Sciences Omics Information Center (http://www.k.u-tokyo.ac.jp/omics/). The results of the analysis were visualized using IGV (Integrative Genome Viewer, Broad Institute; http://software.broadinstitute.org/software/igv/).
RNA isolation, reverse transcription, and quantitative RT-PCR
Plants were cultivated for 10 d under either 0 or 10 μM Mn and were then dissected into shoots and roots. The tissues were disrupted twice at 2000 rpm for 15 s using a multi-bead shocker [MB 755 U (S) type, Yasui Kikai Co., Ltd.]. Total RNA was extracted using NucleoSpin® RNA Plant with rDNase (Macherey-Nagel GmbH & Co. KG).
The extracted RNA was reverse-transcribed into cDNA using a Prime Script RT-PCR Kit (Takara, Japan). The diluted cDNA was applied for quantitative PCR using SYBR®PremixEx TaqII (Takara). Transcripts were detected using the following primers: for NRAMP1, 5′-ATTCATTTCCAGTTCCGGCG-3′ and 5′-CCTGGGTCTGGCTTTGAGTA-3′; For PGSIP6, 5′-CCCA CCATTAGACCCAACCT-3′ and 5′-AGGGATGGCTTTTCT GGCTA-3′.
Plasmid construction and plant transformation
For the complementation test, a genomic fragment containing 2.3 kbp upstream of the first codon of PGSIP6 was amplified by PCR using PrimeSTAR® Max DNA Polymerase (Takara) with primers (5′-CACCCATTGGCACCTGTTGTTGTC-3′ and 5′-ACAGAGGAAACATAGGGAATTTG-3′). PCR products were cloned into pENTR™/D-TOPO® (Life Technologies) and then transferred to a Gateway destination vector pMDC107 (Curtis and Grossniklaus, 2003) using LR clonase. The obtained plasmids were transformed into Agrobacterium tumefaciens GV 3101::pMP90 and then transformed into mutant 30-11 using the floral dip method (Clough and Bent, 1998).
Subcellular observations of PGSIP6
Plants grown under normal Mn conditions for one week were observed using a confocal laser scanning microscope (FV1000, Olympus). The roots were stained with propidium iodide (PI, 10 µg ml–1). For co-localization of the Golgi marker protein ST-mRFP (Ito et al., 2012), F1 plants ST-mRFP/Col-0 (red fluorescent protein) and PGSIP6-GFP/30-11 (green fluorescent protein) were used. GFP, PI, and mRFP fluorescence were observed with the following excitation and emission settings: 488 nm and 505–540 nm band pass filter for GFP, and 559 nm and 570–670 nm for PI and mRFP.
Results
Isolation of mutants 35-34 and 30-11
We screened for low-Mn-sensitive mutants using EMS-mutagenized Arabidopsis seeds, selecting plants with halted or reduced growth under 0 µM Mn compared to growth under normal Mn conditions (10 µM). Through this screening, we obtained eight mutant lines (Supplementary Fig. S1 at JXB online) and from among these, we focused on two mutants, 35-34 and 30-11.
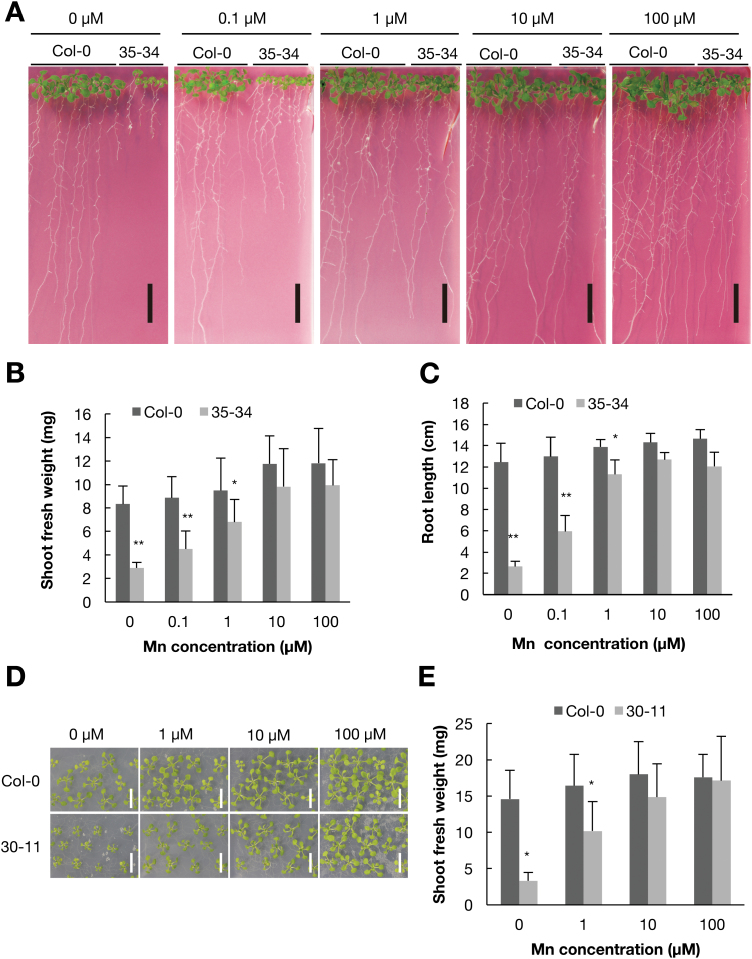
One of the isolated lines, 35-34, showed a short root and small shoot phenotype under 0 μM Mn (Fig. 1A), with the shoot fresh weight being lower than that in the wild-type under low-Mn conditions (0, 0.1, and 1 µM; Fig. 1B). Under normal and high-Mn (100 µM) conditions, the shoot weight was similar to that of the wild-type. The root length of mutant 35-34 showed similar tendencies as observed for shoot weight (Fig. 1C).
Fig. 1.
Low-manganese sensitivity of mutants 35-34 and 30-11. (A) The phenotypes of wild-type Col-0 and mutant 35-34 under various Mn concentrations. Plants were grown on MGRL medium at 22 °C for 14 d. Scale bars indicate 1 cm. (B) Fresh weight of the shoots of Col-0 and mutant 35-34 under different Mn concentrations. (C) The length of the main root of Col-0 and mutant 35-34. (D) The phenotype of Col-0 and mutant 30-11 under various Mn concentrations. Plants were grown on MGRL medium for 14 d. Scale bars indicate 1 cm. (E) Shoot fresh weight of Col-0 and mutant 30-11. Plants were grown under various Mn concentrations for 14 d. Data in (B, C, E) are means (+SD), n=5–8 (B, C), n=9–12 (E). Asterisks indicate significant differences between Col-0 and the mutants as determined by Student’s t-test (*P<0.05, **P<0.01).
Another mutant line, 30-11, showed a small shoot phenotype under low-Mn conditions (Fig. 1D), with the shoot fresh weight being was less than that of Col-0 under 0 and 1 µM Mn (Fig. 1E). Under normal and high-Mn conditions, the shoot fresh weight of mutant 30-11 and the wild-type were comparable. In contrast to 35-34, the root length of mutant 30-11 was comparable to that of the wild-type under both normal and Mn-deficient conditions (data not shown). These results suggested that the causal gene of mutant 30-11 confers low-Mn tolerance to shoots.
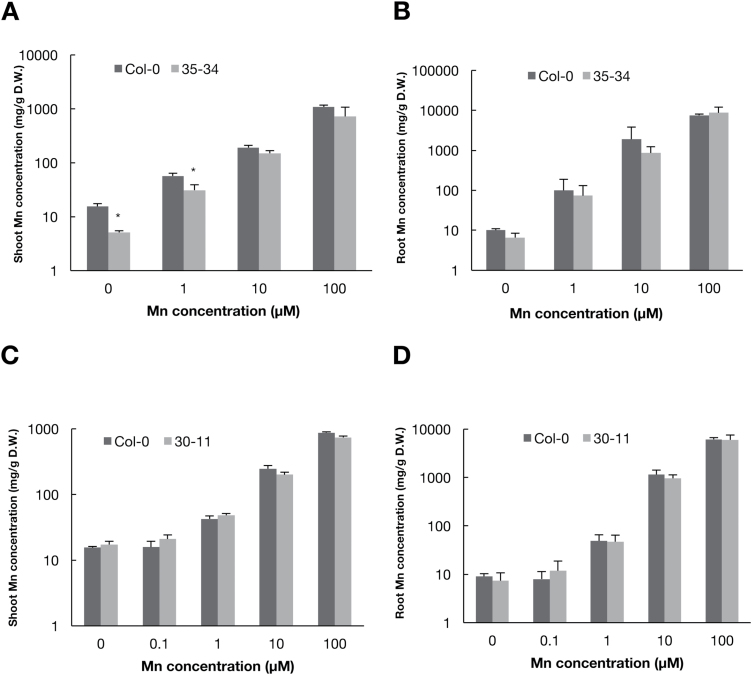
To observe the function of the causal gene, we determined the Mn concentration in the mutants, using inductively coupled plasma mass spectrometry (ICP-MS) after growing plants under various Mn conditions. Under low-Mn conditions (0 and 1 μM), the shoots of 35-34 had lower Mn concentrations than those of Col-0 (Fig. 2A); however, no significant differences were observed for root concentrations (Fig. 2B). This suggested that the causal gene of 35-34 is responsible for Mn transport. The Mn concentrations in shoots and roots of 30-11 were also determined using ICP-MS, and no significant differences were found between the mutant and Col-0, irrespective of Mn concentration in the medium (Fig. 2C, D), suggesting that the causal gene of 30-11 would not be involved in Mn transport.
Fig. 2.
Mn concentrations of wild-type Col-0 and mutants 34–35 and 30-11. Plants were grown for 14 d under various concentrations of Mn. Mutant 35-34 was grown horizontally and mutant 30-11 was grown vertically. Mn concentrations in shoots and roots were determined using ICP-MS and are expressed as means (+SD). Mn concentrations in (A) shoots and (B) roots of mutant 34–35 (n=8). Mn concentrations in (C) shoots and (D) roots of mutant 30-11 (n=8–14). Asterisks indicate significant differences between Col-0 and the mutants as determined by Student’s t-test (*P<0.05).
Identification of the causal gene of mutant 35-34
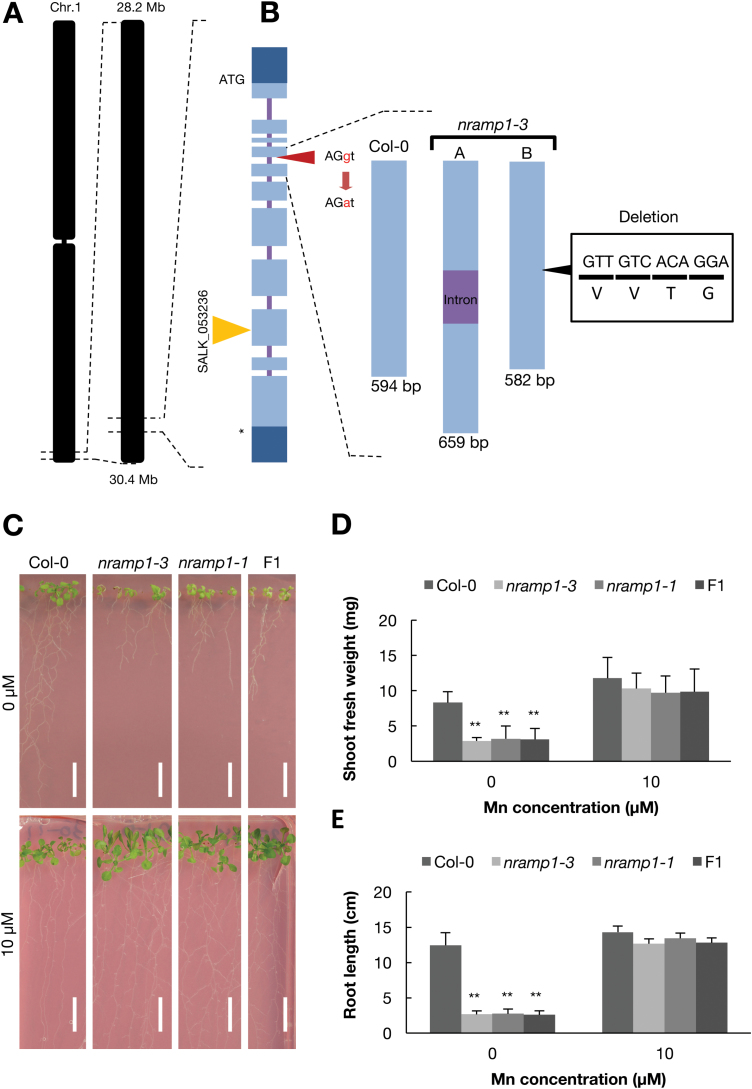
To identify the causal gene of 35-34, we crossed the mutant with Landsberg erecta (Ler) and obtained a selfed F2 population. The segregation ratio of the F2 plants was 241:85 wild-type to mutant phenotype. According to a χ2 test, the segregation ratio was not significantly different from the expected ratio of 3:1 (P=0.65), suggesting that the phenotype of mutant 35-34 is caused by a single recessive mutation. Genetic mapping revealed that the causal gene of the mutant was located between 28.16 and 30.42 Mb on chromosome 1 (Fig. 3A). Based on the TAIR10 database (https://arabidopsis.org), there are 836 genes within this region. Among these, we found a gene encoding a Mn2+ transporter, NRAMP1. It has been reported that the disruption of NRAMP1 makes plants sensitive to low-Mn conditions: shoots are small and etiolated and the root length is short compared with that of the wild-type (Cailliatte et al., 2010).
Fig. 3.
Identification of NRAMP1 as the causal gene. (A) Genetic mapping and the mutation site of nramp1-3, the exon–intron structures of the NRAMP1 gene, and the insertion site of T-DNA in SALK_053236 (nramp1-1). (B) A schematic diagram of the NRAMP1 gene in the wild-type and the splicing junction mutation in nramp1-3. Light blue boxes indicate exons encoding a protein, dark blue boxes are untranslated regions, and the bars between them are introns. ‘A’ represents the intron-inserted transcript and indicates the upper band shown in Supplementary Fig. S2A. ‘B’ represents the exon transcript that has a 12-bp deletion and indicates the bottom band in Supplementary Fig. S2A. (C) Phenotype of wild-type Col-0, nramp1-3, nramp1-1, and F1 plants between nramp1-3 and nramp1-1 under Mn deficiency and normal conditions. Plants were grown for 14 d. Scale bars indicate 1 cm. Fresh weight of shoots (D) and root length (E) of Col-0, nramp1-3, nramp1-1, and F1 plants. Data are means (+SD), n=6. Asterisks indicate significant differences between Col-0 and the mutants as determined by a Tukey–Kramer test (**P<0.01).
We then determined the genomic sequence of NRAMP1 of mutant 35-34 using Sanger sequencing and found a mutation. Therefore, and hereafter, we refer to 35-34 as nramp1-3. The mutation is a nucleotide substitution from a guanine (G) to an adenine (A) at the acceptor site of the splicing junction between the fourth exon and the fourth intron (Fig. 3B). To examine the effects of the mutation in NRAMP1 splicing, we performed PCR using nramp1-3 cDNA as a template. Under both low- and normal Mn conditions, mis-spliced NRAMP1 fragments were present in both shoots and roots (Supplementary Fig. S2A). We then determined the sequences of the mis-spliced fragments and found retention of the fourth intron (nramp1-3a) and a 12-bp deletion [a four-amino-acid (VVTG) deletion] in the fourth exon (nramp1-3b). The intron retention leads to an early stop-codon 51 nucleotides downstream from the fourth exon–intron junction and the addition of 17 amino acids (Supplementary Fig. S2B).
To confirm that the mutation in NRAMP1 was responsible for the low-Mn-sensitivity phenotype of nramp1-3, we obtained a T-DNA insertion line of NRAMP1 (SALK_053236: nramp1-1) (Fig. 3B). When grown under low Mn, nramp1-3 and nramp1-1 showed 2.5-fold less shoot fresh weight and shorter root length than the wild-type; However, no differences were found between the wild-type and mutant plants in the presence of normal Mn (10 μM) (Fig. 3C–E). In addition, we performed an allelism test between the nramp1-3 and nramp1-1 lines. The phenotype of the F1 crosses between the nramp1-3 and nramp1-1 lines was similar to the mutants under low-Mn conditions (0 μM) (Fig. 3C–E). These findings indicate that the causal gene of nramp1-3 is NRAMP1, and hence through this study we obtained a novel nramp1 mutant allele.
Identification of the causal gene of 30-11
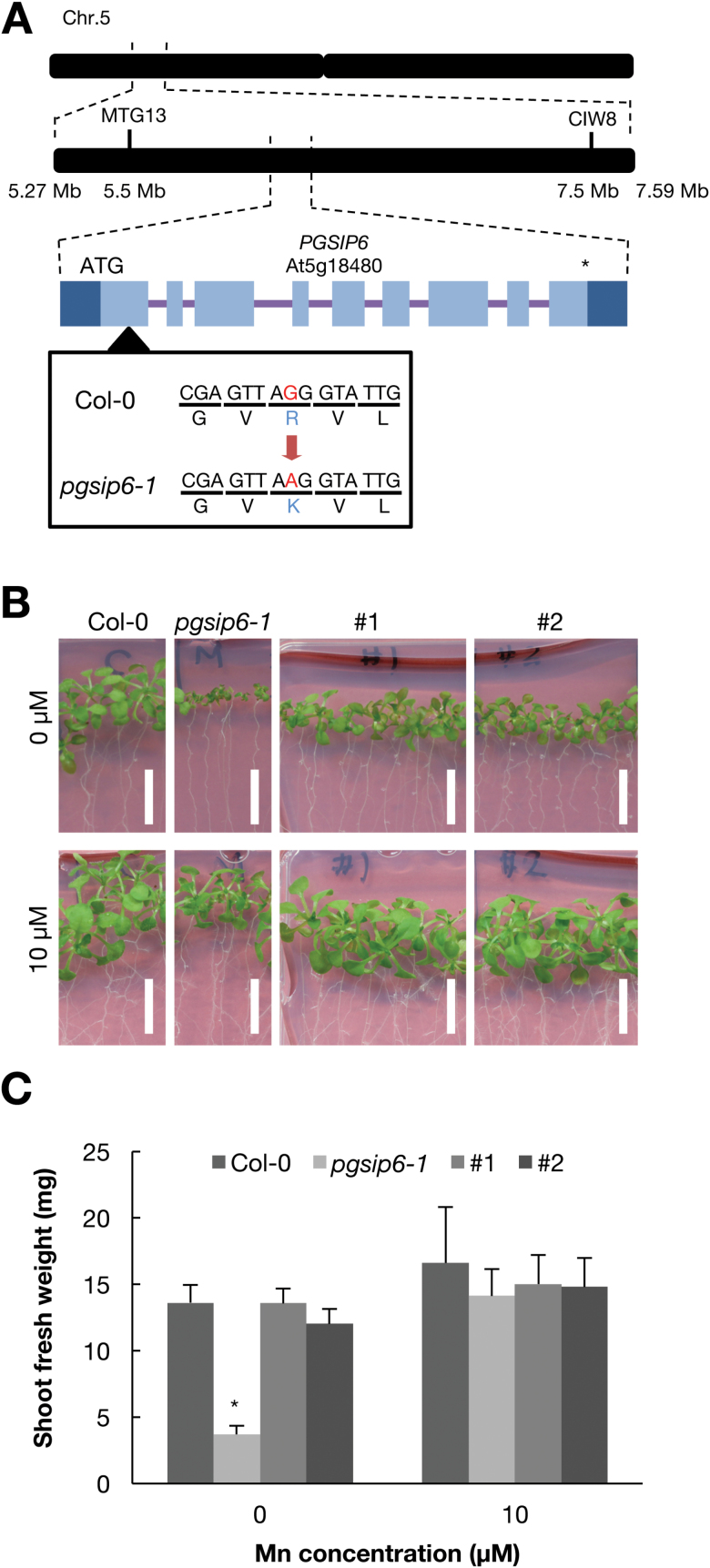
To identify the causal gene of 30-11, the mutant was crossed with Ler and an F2 population was obtained. The F2 seeds were sown on low-Mn medium and the segregation ratio of the wild-type and the mutant was found to be 149:46. The result of a χ2 test (P=0.65) suggested that the low-Mn phenotype is caused by a single recessive mutation. From genetic mapping, the causal gene of 30-11 was found to be located between 5.27 and 7.59 Mb on chromosome 5, which contains 204 genes based on TAIR10 (Fig. 4A). To identify the causal gene, whole-genome sequencing of the mutant was performed using next-generation sequencer SOLiD™. There is only one non-synonymous mutation in this region, which is At5g18480, Plant Glycogenin-like Starch Initiation Protein 6 (PGSIP6). In 30-11, Arg47 is substituted with a Lys. PGSIP6 is predicted to have a Mn2+-binding domain according to UniProt (http://www.uniprot.org; prediction based on Gibbons et al., 2002). Hereafter, we refer to 30-11 as pgsip6-1. PGSIP6 encodes inositol phosphorylceramide glucuronosyltransferase, which is involved in glycosyl inositol phosphorylceramide sphingolipid glycosylation (Tartaglio et al., 2017).
Fig. 4.
Disruption of PGSIP6 causes low-Mn sensitivity. (A) Genetic mapping and the mutation site of mutant 30-11. The markers and their positions are according to the TAIR10 database. Light blue boxes indicate exons encoding a protein, dark blue boxes are untranslated regions, and the bars between them are introns. MTG13 and GIW8 indicate the markers. (B) Complementation of PGSIP6 with a construct in which genomic PGSIP6 DNA with the promoter region was fused with GFP. Wild-type Col-0, pgsip6-1, and two complementation lines were grown under low-Mn and normal conditions for 14 d. Scale bars indicate 1 cm. (C) Shoot fresh weight of Col-0, pgsip6-1, and two complementation lines of PGSIP6 (#1 and #2). Asterisks indicate significant differences between mutant 30-11 and the two complementation lines as determined by a Tukey–Kramer test (*P<0.05).
To test whether PGSIP6 was the causal gene, we performed complementation analysis since a T-DNA line was not available. A genomic fragment of PGSIP6 fused with GFP (PGSIP6-GFP) was introduced into the mutant pgsip6-1. The phenotypic defect of pgsip6-1 was rescued by PGSIP6-GFP (Fig. 4B, C). These results indicate that PGSIP6 is the causal gene of pgsip6-1.
Expression analysis of PGSIP6
The mRNA expression levels of PGSIP6 in the shoots and roots of the wild-type under low- and normal Mn conditions were measured using real-time PCR. There were no significant differences either in the shoots or roots under low-Mn conditions compared to normal conditions (Supplementary Fig. S3).
PGSIP6 is localized to the Golgi apparatus
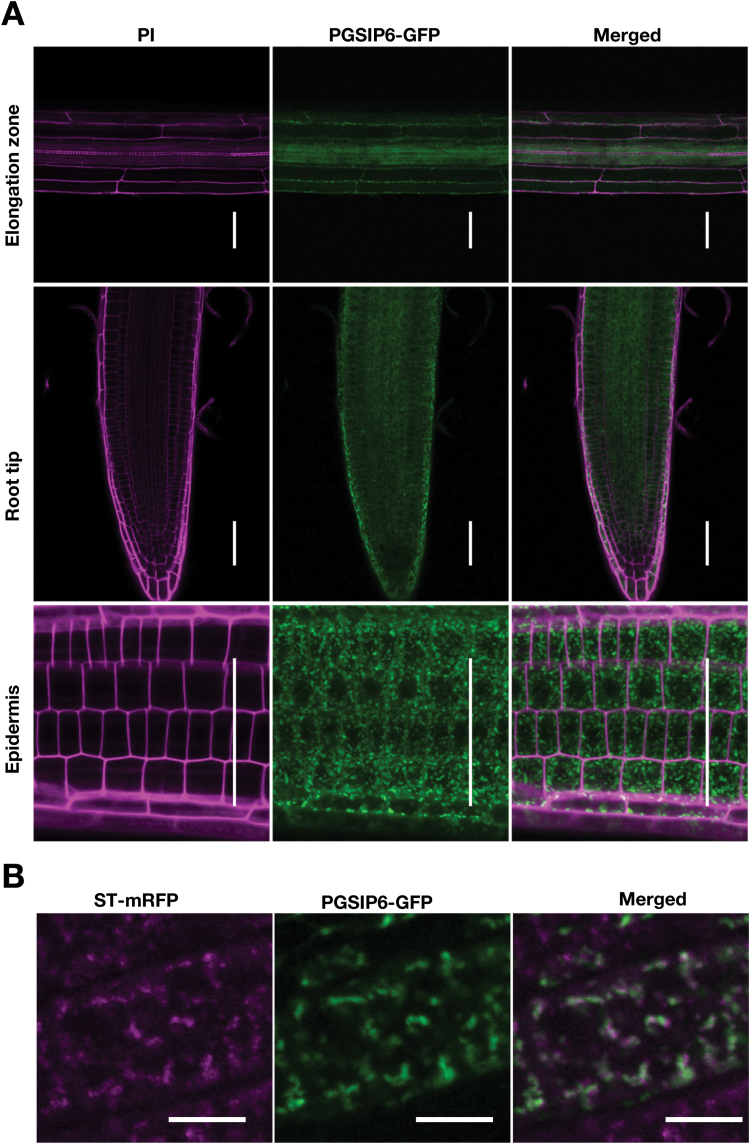
To identify tissue-specific expression and subcellular localization, we observed PGSIP6-GFP fluorescence of the complemented line (Fig. 4B). GFP fluorescence was widely observed in roots (Fig. 5A), being seen as dots, and it was partially co-localized with the trans-Golgi marker ST-mRFP (Ito et al., 2012) (Fig. 5B), which is consistent with observations in a previous study after transient expression in tobacco epidermal cells (Rennie et al., 2012; Nikolovski et al., 2014). Some of the dots were not co-localized with ST-mRFP. Considering that ST-mRFP is localized to the trans-Golgi compartment, PGSIP6-GFP may be localized to the cis-Golgi as well.
Fig. 5.
The expression and localization of PGSIP6-GFP in root cells. (A) Expression of the PGSIP6-GFP fusion protein in root tissues. Transformed plants expressing PGSIP6-GFP were observed using confocal microscopy. The cell wall was stained with propidium iodide (PI). Plants were grown on MGRL medium for 5 d. Scale bars indicate 50 μm. (B) Subcellular localization of the PGSIP6-GFP fusion protein in root cells. The fluorescence of PGSIP6-GFP was imaged with the Golgi marker ST-mRFP under normal Mn conditions. Golgi marker ST-mRFP fluorescence (left), GFP (middle), and merged images (right) are shown. Plants were grown on MGRL medium for 7 d and observed by confocal laser microscopy. Scale bars indicate 10 μm.
Discussion
In this study, we isolated eight low-Mn-sensitive mutants (Supplementary Fig. S1). We focused on two of them, nramp1-3 and pgsip6-1, and identified two new alleles of NRAMP1 and PGSIP6 with altered low-Mn sensitivity, probably due to perturbation of the intracellular distribution and utilization of Mn, respectively.
Effect of the nramp1-3 mutation in NRAMP1 function
In this study, we found that nramp1-3 has a mutation at an exon–intron junction that produces two different transcripts, either with the intron retained or with a 12-bp deletion (Fig. 3B). Two transcripts in nramp1-3 could be produced, because one transcript with the intron led to a premature termination codon 51 bp downstream from the exon–intron junction, resulting in the addition of 17 amino acids (Supplementary Fig. S2B). The other transcript led to the loss of four amino acids in the second transmembrane domain, which is highly conserved among various plant species (Supplementary Figs S2B, S4). We confirmed that the phenotypes of nramp1-3 were similar to the nramp1-1 line. These data suggest that the low-Mn-sensitive phenotype of nramp1-3 is due to the abnormal splicing of NRAMP1.
Mn concentration-dependent phenotypes of pgsip6-1
pgsip6-1 showed Mn-dependent phenotypes. One possibility is that mutated PGSIP6 has a low affinity for Mn2+ compared to the wild-type. PGSIP6 has a nucleotide-diphospho-sugar transferase domain (Campbell et al., 1997) in its N-terminus and a transmembrane domain in its C-terminal region (Supplementary Fig. S5). pgsip6-1 has a mutation in the N-terminal domain, where the Mn2+-binding site is predicted based on UniProt (http://www.uniprot.org). This prediction is based on the crystal structure of rabbit glycogenin (Gibbons et al., 2002), in which three amino acid residues (Asp101, Asp103, and His211) directly bind with Mn2+. All of the amino acids are conserved in PGSIP6 (Asp114, Asp116, and His248) and also in other homologous genes (Supplementary Fig. S5). In addition, Rennie et al. (2012) showed that GUX1, the protein most similar to PGSIP6, requires Mn2+ for its activity. Therefore, PGSIP6 could require Mn2+ for its activity. The mutation site in pgsip6-1 is Arg47, which is not close to the Mn2+-binding site. Therefore, pgsip6-1 may result in a partial loss in PGSIP6 enzymatic activity, leading to the Mn2+-dependent phenotype. If the mutation caused more than a partial loss of PGSIP6 activity and reduced it to a large extent, then the alternative glucoronosylation pathway that is strongly dependent on Mn2+ would be exposed, resulting in the Mn2+-dependent phenotype.
Another possibility is that the product of PGSIP6 determines the Mn tolerance of plants. PGSIP6 is an inositol phosphorylceramide (IPC) glucuronosyltransferase (Rennie et al., 2014) that produces glycosyl inositol phosphorylceramide (GIPC). Recent reports have indicated that GIPCs are a major component of plant plasma membranes and important for the maintenance of membrane microdomains (Markham et al., 2006; Cacas et al., 2016). However, to date there are no reports about the function of Mn2+ in relation to microdomain maintenance.
Until now, there has been no mutant allele available for PGSIP6. In the T-DNA heterozygous line, mutant pollen was found to be defective at later stages of reproduction (Rennie et al., 2014; Tartaglio et al., 2017). Our mutant could provide good material for functional analyses of PGSIP6 and its product, GIPC.
In conclusion, our study has shown that NRAMP1 and PGSIP6 are important for plant survival under low-Mn conditions. NRAMP1 functions as a Mn transporter and is involved in low-Mn tolerance. PGSIP6 is a Mn2+-binding glucuronosyltransferase that is associated with GIPC sphingolipid glycosylation. This study provides new insights to GIPC sphingolipid glycosylation, mediated by PGSIP6, and connects it to adaptation to low-Mn conditions.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Phenotypes of various low-Mn-sensitive mutants.
Fig. S2. Splicing variants of nramp1-3.
Fig. S3. Expression of PGSIP6 under 0 and 10 µM Mn conditions.
Fig. S4. Multiple amino acid sequence alignment of NRAMPs.
Fig. S5. Multiple amino acid sequence alignment of PGSIPs.
Acknowledgments
We thank the ABRC for providing the materials used in this study. We also thank Emiko Yokota for providing technical assistance. This work was supported by JSPS KAKENHI Grant Numbers 26712008 (to TK), and 15H01224 and 25221202 (to TF).
Glossary
Abbreviations:
- NRAMP
natural resistance-associated macrophage protein
- GIPC
glycosyl inositol phosphorylceramide
- PGSIP
plant glycogenin-like starch initiation protein.
References
- Agorio A, Giraudat J, Bianchi MW, Marion J, Espagne C, Castaings L, Lelièvre F, Curie C, Thomine S, Merlot S. 2017. Phosphatidylinositol 3-phosphate–binding protein AtPH1 controls the localization of the metal transporter NRAMP1 in Arabidopsis. Proceedings of the National Academy of Sciences, USA 114, E3354–E3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both P, Sobczak L, Breton C, Hann S, Nöbauer K, Paschinger K, Kozmon S, Mucha J, Wilson IB. 2011. Distantly related plant and nematode core α1,3-fucosyltransferases display similar trends in structure–function relationships. Glycobiology 21, 1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Buré C, Grosjean K, et al. 2016. Revisiting plant plasma membrane lipids in tobacco: a focus on sphingolipids. Plant Physiology 170, 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. 2010. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. The Plant Cell 22, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Davies GJ, Bulone V, Henrissat B. 1997. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. The Biochemical Journal 326, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier M, Prive G, Belouchi A, Kwan T, Rodrigues V, Chia W, Gros P. 1995. Nramp defines a family of membrane proteins. Proceedings of the National Academy of Sciences, USA 92, 10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE. 2007. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. The Plant Journal 51, 198–210. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S. 1992. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiology 99, 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Xie W, Yang C, Xu J, Li J, Wang H, Chen X, Huang CF. 2018. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytologist 217, 179–193. [DOI] [PubMed] [Google Scholar]
- Gibbons BJ, Roach PJ, Hurley TD. 2002. Crystal structure of the autocatalytic initiator of glycogen biosynthesis, glycogenin. Journal of Molecular Biology 319, 463–477. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Takahashi R, Bashir K, et al. 2012. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Scientific Reports 2, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Shoda K, Fujimoto M, Ueda T, Nakano A. 2012. cis-Golgi proteins accumulate near the ER exit sites and act as the scaffold for Golgi regeneration after brefeldin A treatment in tobacco BY-2 cells. Molecular Biology of the Cell 23, 3203–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Smith AP, Nagarajan VK, Lahner B, Meagher RB, Raghothama KG. 2009. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiology 150, 1033–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata-Pendias A. 2011. Trace elements in soils and plants. Boca Raton, FL: CRC Press. [Google Scholar]
- Kern J, Loll B, Zouni A, Saenger W, Irrgang KD, Biesiadka J. 2005. Cyanobacterial photosystem II at 3.2 Å resolution – the plastoquinone binding pockets. Photosynthesis Research 84, 153–159. [DOI] [PubMed] [Google Scholar]
- Lanquar V, Ramos MS, Lelièvre F, Barbier-Brygoo H, Krieger-Liszkay A, Krämer U, Thomine S. 2010. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiology 152, 1986–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JE, Li J, Cahoon EB, Jaworski JG. 2006. Separation and identification of major plant sphingolipid classes from leaves. The Journal of Biological Chemistry 281, 22684–22694. [DOI] [PubMed] [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants. Boston, MA: Academic Press. [Google Scholar]
- Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE. 2008. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiology 146, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y, Nelson N. 2006. The NRAMP family of metal-ion transporters. Biochimica et Biophysica Acta 1763, 609–620. [DOI] [PubMed] [Google Scholar]
- Nikolovski N, Shliaha PV, Gatto L, Dupree P, Lilley KS. 2014. Label-free protein quantification for plant Golgi protein localization and abundance. Plant Physiology 166, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S. 2008. Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiology 148, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Ebert B, Miles GP, et al. 2014. Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. The Plant Cell 26, 3314–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Hansen SF, Baidoo EE, Hadi MZ, Keasling JD, Scheller HV. 2012. Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant Physiology 159, 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SB, Jensen PE, Husted S. 2016. Manganese deficiency in plants: the impact on photosystem II. Trends in Plant Science 21, 622–632. [DOI] [PubMed] [Google Scholar]
- Socha AL, Guerinot ML. 2014. Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Frontiers in Plant Science 5, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Vavra U, et al. 2007. A unique β1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. The Plant Cell 19, 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata R, Kamiya T, Shigenobu S, Yamaguchi K, Yamada M, Hasebe M, Fujiwara T, Sawa S. 2013. Identification of an EMS-induced causal mutation in a gene required for boron-mediated root development by low-coverage genome re-sequencing in Arabidopsis. Plant Signaling & Behavior 8, e22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglio V, Rennie EA, Cahoon R, Wang G, Baidoo E, Mortimer JC, Cahoon EB, Scheller HV. 2017. Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidopsis. The Plant Journal 89, 278–290. [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen JR, Kamiya N. 2011. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. 2002. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell 14, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TJ, Perry PJ, Ciani S, Pandian S, Schmidt W. 2008. Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. Journal of Experimental Botany 59, 3453–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.