Key Clinical Message
Patent Blue dye for sentinel lymph node biopsy is associated with systemic allergic response and generalized blue hue. We report a unique case of successful free flap transfer in this setting. Despite inotropic support and abnormal blue hue, allergic response does not preclude safe flap transfer and monitoring.
Keywords: DIEP, free flap, lymph node, microsurgery, microsurgical, sentinel node
Introduction
Patent Blue V dye is widely used in the identification of sentinel lymph nodes in patients with breast cancer. Anaphylaxis to Patent Blue V dye ranges from 06% to 2.7%. Blue hives and a generalized blue hue have rarely been reported. We report a unique case of a deep inferior epigastric perforator free flap transferred in the setting of a generalized blue hue allergic response. The flap maintained a pink color despite surrounding blue chest wall skin, and monitoring was not problematic despite the risk of being so. This case highlights the intraoperative vigilance required while administering Patent Blue V dye. We recommend the use of alternative dyes such as Methylene Blue if anaphylaxis is of concern, and we recommend surgeons to discuss the allergic risks involved with Patent Blue V dye during the consent process, including those related to monitoring a free flap reconstruction.
Patent Blue V dye (PBVD) has been extensively used for sentinel lymph node biopsy (SNB) 1, 2. The use of this dye is associated with local blue skin and deeper tissue discoloration, and systemic absorption can lead to altered color of body secretions. Anaphylaxis to PBVD ranges from 06% to 2.7%, and allergic reactions can lead to a phenomenon of “blue hives,” and in addition, a generalized blue hue has rarely been reported 3.
Nonallergic reactions to PBVD include skin tattooing and a localized bluish hue at the injection site persisting for a few hours 2, 4. These adverse effects can have negative impacts on breast reconstructive surgery. To our knowledge, there has been one reported incidence of local skin necrosis following PBVD injection. This patient underwent SNB, skin‐sparing mastectomy, and immediate reconstruction with a latissimus dorsi flap for a ductal carcinoma in situ 5. The effects of Patent Blue dye and reactions to it have not been reported in the setting of free flap breast reconstruction, and the effects on microsurgical outcomes are unknown.
Case Report
We present a 65‐year‐old woman who underwent a left breast skin‐sparing mastectomy, left internal mammary and right axillary sentinel lymph node biopsy, and immediate deep inferior epigastric perforator (DIEP) free flap reconstruction for a second primary breast cancer. Her significant past medical history included previous left breast cancer treated with wide local excision, hysterectomy, and hypertension. She had no previous adverse reactions to anesthesia and no previous exposure to Patent Blue or other dyes.
Patent Blue dye (PBD) was injected subcutaneously into the superior quadrants of the left breast. One ampule (2 mL) was injected, as per institutional guidelines. The 6 mm tumor was located at the 2 o'clock position 5 cm from the nipple. The DIEP flap was concurrently being raised from its abdominal donor site by the reconstructive team. Approximately 10 min after the dye was injected, the patient developed progressive tachycardia (110 bpm) followed by hypotension (systolic blood pressure fell from 130 mmHg to 70 mmHg) and bradycardia (40 bpm). The patient's oxygen saturation dropped from 100% to 94% on 60% FiO2. A diagnosis of systemic allergic response was made on clinical grounds (no investigations such as serum tryptase were used). This was treated immediately as per institutional guidelines, with intravenous adrenaline and metaraminol for blood pressure control. Throughout the operation, the patient received multiple doses of adrenaline and subsequently required an adrenaline infusion (250–500 mcg/h) to maintain her blood pressure. These events were due to an anaphylactic reaction to Patent Blue dye. The patient was stabilized, and given that the DIEP flap had been partially raised, a combined decision was made to proceed with both cancer resection and reconstruction. The DIEP flap was raised and anastomosed to the left internal thoracic artery and vein.
During the operation, approximately two hours after injecting Patent Blue into the breast, the patient developed a diffuse bluish discoloration of her upper body from the umbilicus (including upper limbs) to her face. The blue tinge spared the DIEP flap (see Fig. 1). After flap transfer, the pale/pink DIEP flap was in stark contrast to the surrounding blue chest wall, providing a unique experience for flap monitoring. Postoperatively, the patient was admitted to the intensive care unit (ICU) for a period of inotropic support and monitoring, but ultimately was discharged from hospital and she had a good reconstructive outcome. There were no operative or perioperative flap‐related complications, specifically as a result of the allergic reaction to the Patent Blue dye.
Figure 1.
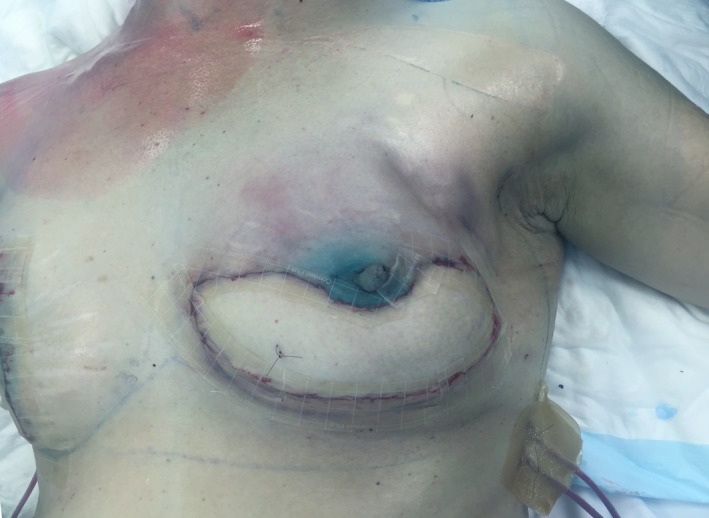
Generalized blue hue following allergic response to Patent Blue dye, with relative sparing of the transferred deep inferior epigastric artery (DIEP) flap for breast reconstruction.
Discussion
Patent Blue is widely used in sentinel lymph node biopsy identification, and allergic responses are rare but widely reported. The incidence of anaphylaxis ranges from 0.6% to 2.7% 3. The allergic reactions of PBVD that have been reported in the literature can be classified by grades 2:
Grade I–Urticaria, blue hives, pruritus, or generalized rash.
Grade II–Transient hypotension/bronchospasm/laryngospasm.
Grade III–Severe hypotension (requiring vasopressor support) and/or change/abandoning of planned procedure, and/or HDU/ITU admission.
Grade IV–Cardiorespiratory arrest and/or death.
Systemic blue discoloration of skin has been previously reported in the literature 6, 7, 8, 9. In all these cases, Patent Blue dye was injected subcutaneously. The forms of blue discoloration included blue urticaria and blue angioedema. In all these patients, it was deemed they had an allergic reaction has caused the discoloration. Our patient did not display any urticaria or angioedema. She displayed symptoms of anaphylaxis and blue discoloration only above (cranial to) the level of her umbilicus. The blue discoloration only lasts for several hours in the setting of blue hives in the literature, and this was also true for our patient. In our patient, the dye was injected into the subcutaneous plane and the dye was then absorbed into the lymphatics and hence into the venous circulation 10. An important implication of this systemic discoloration is that it can produce erroneous pulse oximetry readings and create issues with flap monitoring 10.
Other dyes have been used in SNB. Of the alternatives to Patent Blue, Methylene Blue dye and Isosulfan Blue (“Lymphazurin”) dye (which are predominately used in the United States) are the more frequent to be used in SNB. It has been reported that Methylene Blue dye may have caused severe capsular contraction in association with intense Methylene Blue dye staining of a saline‐filled prosthesis. This occurred following immediate reconstructive surgery for breast cancer 11. Local skin reactions to Methylene Blue dye injection have also occurred, including skin and fat necrosis and skin infection 12, 13, 14. There has been a reported incidence of Isosulfan Blue causing a delay in a planned immediate transverse rectus abdominis myocutaneous flap reconstruction for breast cancer. The reconstruction was delayed due to the patient developing widespread blue urtica and hives making it difficult to assess the tissue perfusion 15. In addition, there is antibody cross‐reactivity between Isosulfan Blue and Patent Blue but not Methylene Blue. In cases of Patent Blue hypersensitivity, Methylene Blue may be used as an alternative 13, 16, 17. While we use radioisotope scanning (lymphoscintigraphy) as an adjunct to Patent Blue, it can be used in isolation where the risk of Patent Blue V allergy is suspected. The risk of allergic reaction to these is negligible.
A recent article suggested only 53.3% of surgeons discussed the risks of PBVD allergies as part of their informed consent 1. The same article recommends confirmatory testing should be carried out to ascertain the causative agent 1. This case has highlighted the complications of PBVD. In fact, the PBVD product information insert recommends testing for hypersensitivity by injecting a small volume of solution initially, then awaiting a short time to see whether an allergic reaction develops 10.
Conclusions
Patent Blue dye for sentinel lymph node biopsy is associated with systemic allergic response and generalized blue hue. We have highlighted a case of successful free flap transfer in this setting. Despite inotropic support and abnormal blue hue, allergic response did not preclude safe flap transfer and monitoring, and proceeding with flap transfer in this setting can be safe.
In patients with known hypersensitivity to PBVD, however, we recommend the use of Methylene Blue dye as an alternative, as suggested in the broader literature 13, 16. We also recommend the allergic reactions of PBVD be discussed during the consent process. Given that we were unable to identify any evidence to suggest PBVD is detrimental to reconstructive surgery, we recommend the continued use of PBVD with the above recommendations.
Conflict of Interest
None declared.
Authorship
SK: reviewed the literature and involved in writing. RD: was the Operating Surgeon and involved in writing. SS: involved in writing and contributed to review. DCW: involved in writing, contributed to review, and involved in supervision. WMR: was the Operating Surgeon, involved in writing, and contributed to review.
Clinical Case Reports 2018; 6(4): 581–584
References
- 1. Wong, A. , and Spillane A.. 2014. Breast Surgeons of A, New Zealand I. Patent Blue V dye anaphylaxis: experience of Australian and New Zealand surgeons. ANZ J. Surg. 84:37–41. [DOI] [PubMed] [Google Scholar]
- 2. Barthelmes, L. , Goyal A., Newcombe R. G., McNeill F., and Mansel R. E.. 2010. Adverse reactions to Patent Blue V dye–The NEW START and ALMANAC experience. Eur. J. Surg. Oncol. 36:399–403. [DOI] [PubMed] [Google Scholar]
- 3. Scherer, K. , Studer W., Figueiredo V., and Bircher A. J.. 2006. Anaphylaxis to isosulfan blue and cross‐reactivity to Patent Blue V: case report and review of the nomenclature of vital blue dyes. Ann. Allergy Asthma Immunol. 96:497–500. [DOI] [PubMed] [Google Scholar]
- 4. Govaert, G. A. , Oostenbroek R. J., and Plaisier P. W.. 2005. Prolonged skin staining after intradermal use of Patent Blue in sentinel lymph node biopsy for breast cancer. Eur. J. Surg. Oncol. 31:373–375. [DOI] [PubMed] [Google Scholar]
- 5. Jaffer, U. , Badri H., and Abdullah T. I.. 2008. Skin necrosis following Patent Blue v injection for sentinel node detection during breast cancer excision: case report. Breast J. 14:508–509. [DOI] [PubMed] [Google Scholar]
- 6. Weng, P. W. , Hsu H. M., Chen T. W., Hsieh C. B., Chang T. M., and Chen V.. 2007. YU JC. Blue angioedema of eyelip after Patent Blue injection for lymphatic mapping procedure. Eur. J. Cancer Care 16:390–391. [DOI] [PubMed] [Google Scholar]
- 7. Manson, A. L. , Juneja R., Self R., Farquhar‐Smith P., MacNeill F., and Seneviratne S. L.. 2012. Anaphylaxis to Patent Blue V: a case series. Asia Pac. Allergy 2:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haque, R. , Wagner A., Whisken J., Nasser S., and Ewan P.. 2010. Anaphylaxis to Patent Blue V: a case series and proposed diagnostic protocol. J. Allergy Clin. Immunol. 65:396–400. [DOI] [PubMed] [Google Scholar]
- 9. Joshi, M. , Hart M., Ahmed F., and McPherson S.. 2012. Adverse reaction; Patent Blue turning patient blue. BMJ Case Rep. 2012:bcr2012007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yusim, Y. , Livingstone D., and Sidi A.. 2007. Blue dyes, blue people: the systemic effects of blue dyes when administered via different routes. J. Clin. Anesth. 19:315–321. [DOI] [PubMed] [Google Scholar]
- 11. Singh‐Ranger, G. , and Mokbel K.. 2004. Capsular contraction following immediate reconstructive surgery for breast cancer–An association with Methylene Blue dye. Int. Semin. Surg. Oncol. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reyes, F. J. , Noelck M. B., Valentino C., Grasso‐LeBeau L., and Lang J. E.. 2011. Complications of Methylene Blue Dye in Breast Surgery: Case Reports and Review of the Literature. J. Cancer 2:20–25. [PMC free article] [PubMed] [Google Scholar]
- 13. Thevarajah, S. , Huston T. L., and Simmons R. M.. 2005. A comparison of the adverse reactions associated with isosulfan blue versus Methylene Blue dye in sentinel lymph node biopsy for breast cancer. Am. J. Surg. 189:236–239. [DOI] [PubMed] [Google Scholar]
- 14. Baker, J. J. , Ollila D. W., Deal A. M., Frank J., Amos K. D., and Meyers M. O.. 2012. Early recurrence in sentinel lymph node positive stage III melanoma patients. Am. Surg. 78:808–813. [PubMed] [Google Scholar]
- 15. Sadiq, T. S. , Burns W. W., Taber D. J., Damitz L., and Ollila D. W.. 2001. Blue urticaria: a previously unreported adverse event associated with isosulfan blue. Arch. Surg. 136:1433–1435. [DOI] [PubMed] [Google Scholar]
- 16. Van Zuuren, E. , Polderman M. C., and Kuijken I.. 2005. Anaphylaxis to Patent Blue during sentinel lymph node identification. Contact Dermatitis 53:171. [DOI] [PubMed] [Google Scholar]
- 17. Soni, M. , Saha S., Korant A., Fritz P., Chakravarty B., Sirop S., et al. 2009. A prospective trial comparing 1% lymphazurin vs 1% Methylene Blue in sentinel lymph node mapping of gastrointestinal tumors. Ann. Surg. Oncol. 16:2224–2230. [DOI] [PubMed] [Google Scholar]


