ABSTRACT
Monoclonal antibodies (mAbs) that block the programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) receptors are the most clinically advanced tumor immunotherapies. Given the broad antitumor efficacy and novel mechanism of action, numerous combinatorial approaches incorporating PD-1/PD-L1 blockade have been suggested; herein we present a comprehensive analysis of these clinical trials. We queried clinicaltrials.gov for all PD-1/PD-L1 mAbs administered for cancer therapy with an end date of 4/30/2017. A total of 1,218 clinical trials met our search criteria. These trials have a planned enrollment of 227,190 patients, and approximately half (493) were initiated in 2016 alone. Of these over 1,200 trials, 916 combine PD-1/PD-L1 blockade with at least one additional therapy, ranging from traditional treatment modalities like surgery and chemoradiation to newer therapies like small molecule inhibitors and other immunotherapies. The staggering proliferation of clinical trials combining PD-1/PD-L1 blockade with disparate treatments necessitates careful accounting to maximize efficiency and highlight areas of unmet needs. We believe our analysis provides this data and expect it will facilitate the design of future clinical trials in this burgeoning area of oncology research.
KEYWORDS: Cancer immunotherapy, clinical trials, PD-1, PD-L1, T cell checkpoint inhibitors
Introduction
Blockade of the programmed death-1/programmed death-ligand-1 (PD-1/PD-L1) pathway has produced remarkable clinical results in the treatment of a wide array of cancers, leading to numerous FDA approvals and hundreds of clinical trials.4 Despite this success, the majority of cancer patients who receive PD-1/PD-L1 blockade will not respond. This fact has led investigators to prioritize development of predictive biomarkers and identification of more efficacious combinatorial therapies.5
In this clinical trial review, we first overview all ongoing oncology clinical trials using PD-1/PD-L1 blocking monoclonal antibodies (mAbs). We then analyze the nearly 1,000 combination clinical trials we identified, dividing them by type of combined therapy: surgery, radiotherapy (RT), chemotherapy, tumor-targeted therapy and immunotherapy.
Results
Summary of all ongoing PD-1/PD-L1 trials
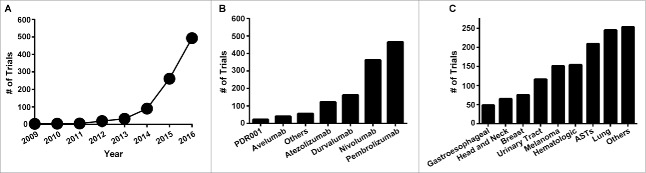
In October 2016 an analysis identified approximately 800 ongoing PD-1/PD-L1 clinical trials.4 Approximately 6 months since this publication, an additional 400 trials have been posted, for a total of 1,218 clinical trials with a planned enrollment of 227,190 patients. The majority of these trials (804) have a listed start date of 2016 or 2017. In fact, the rate of trial initiation is growing exponentially, with a doubling time of 0.9 years (Fig. 1A, R2 = 0.993). With such a large number of trials it is not surprising that they encompass several PD-1/PD-L1 mAbs (Fig. 1B) and dozens of tumor types (Fig. 1C). Given that over 200,000 patients are planned to be enrolled on these trials, it is likewise unsurprising that there are 139 phase 3 and 720 phase 2 trials. Finally, 190 trials are enrolling treatment-naïve patients, a fact that highlights the rapid clinical ascension of these agents from experimental to first-line in a few short years.
Figure 1.
Overview of PD-1/PD-L1 trials. A, number of trials initiated by year. B, number of trials by antibody. C, number of trials initiated by site of tumor. ASTs = advanced solid tumors.
Having identified all ongoing PD-1/PD-L1 mAb cancer trials, we next selected for those that combine these mAbs with another treatment modality in at least one of the arms. Accordingly, multi-arm trials comparing PD-1 monotherapy to other treatments were not included in combination therapy analysis. Even with this additional criterion, 916 of the 1,218 trials combine PD-1/PD-L1 blockade with at least 1 additional therapy (Fig. 2A). These trials include the traditional modalities of surgery (49), radiation (138) and chemotherapy (216) as well as tumor-targeted therapies (261) and other immunotherapies (446, Fig. 2B). The remainder of this analysis will focus on these combination trials, divided broadly according to treatment type.
Figure 2.
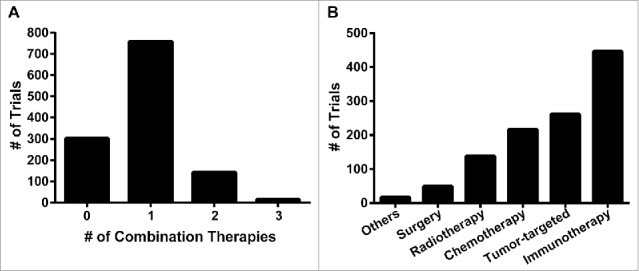
Overview of combination PD-1/PD-L1 trials. A, number of trials listed according to number of therapies combined with PD-1/PD-L1 blockade (e.g. trials with 2 combination therapies add 2 additional agents to PD-1/PD-L1 blockade). B, number of combination trials grouped broadly by treatment modality/mechanism of action.
Surgery
Surgery has long been hypothesized to possess immunomodulatory properties as debulking is thought to remove the immunosuppressive tumor burden and consequently enable immune activation.6 Clinical trials in metastatic renal cell carcinoma (RCC), which demonstrated improved survival when surgery was added to immunotherapy,7 support this concept. More recently, the CTLA-4 blocker ipilimumab, another T cell checkpoint blocking mAb, was FDA approved as a post-surgical adjuvant for stage III melanoma. These results, among others, established a clinical rationale for adding PD-1/PD-L1 blockade to surgical treatment regimens.
Our analysis identified 49 studies (Table 1), 44 of which are in the setting of first-line treatment and 6 of which are phase 3 trials. These studies cover multiple sites, with 9 different tumor histologies having at least 3 ongoing trials (Table 1). Although the evidence cited above employs immunotherapy in the adjuvant setting, the majority of the current studies use PD-1/PD-L1 mAbs in the neoadjuvant setting, with only 5 studies using solely adjuvant PD-1/PD-L1 blockade (Table 1). These neoadjuvant studies, in which PD-1/PD-L1 mAb-treated tissue will be available, should provide invaluable insight into the mechanism of these mAbs in various tumor types.
Table 1.
Ongoing clinical trials combining surgery with PD-1/PD-L1 blockade. Abbreviations: HNSCC = head and neck squamous cell carcinoma, NSCLC = non-small cell lung cancer, RCC = renal cell carcinoma.
Radiotherapy
Radiotherapy (RT), like surgery, has traditionally been considered a local treatment option. This limited role has been evolving as several recent studies have demonstrated that local RT can produce out-of-field clinical responses, a phenomenon termed the abscopal effect. Mechanistically, the abscopal effect is thought to be mediated at least in part by radiation-induced immunogenic cell death (ICD), which leads to an antitumor T cell response.12 Therefore, RT has been hypothesized to provide an in situ vaccine that can either initiate or further propagate an immune response.
We identified 138 studies combining RT with PD-1/PD-L1 blockade, a sizable increase from the 56 trials identified in a similar analysis with May 1, 2016 as end search date. Interestingly, nearly half of the 138 trials are being conducted with pembrolizumab (67 of 138). As expected from the conflicting data regarding the optimal dose and fractionation schemes to use for this combination, the RT approaches being employed vary widely (Table 2). Conventional fractionation, with and without chemotherapy, accounts for 55 of the trials while stereotactic body RT (SBRT) and stereotactic radiosurgery (SRS) combine for 49 trials (Table 2). Although numerous tumor sites are represented, head and neck cancers have 19 ongoing trials using conventional approaches while 13 of the 43 ongoing SBRT trials are enrolling NSCLC patients (Table 2). Finally, in the 6 phase 3 trials identified (NCT02617589, NCT02768558, NCT02926196, NCT02952586, NCT02999087, NCT03040999), all 6 are chemoRT trials with initiation dates of 2016 or 2017, and 3 of these trials are in head and neck cancers.
Table 2.
Ongoing clinical trials combining RT with PD-1/PD-L1 blockade.
Some trials are enrolling multiple tumor types and were listed twice. Abbreviations: CRC = colorectal carcinoma, EBRT = external beam radiotherapy, HNSCC = head and neck squamous cell carcinoma, NSCLC = non-small cell lung cancer, RCC = renal cell carcinoma, RT = radiotherapy, SRS = stereotactic radiosurgery.
Chemotherapy
The importance of the immune system in the efficacy of cytotoxic chemotherapy has long been underappreciated. In recent years, however, its role is becoming more widely recognized. Like RT, certain chemotherapeutics enhance the immunogenicity of tumor cells by inducing ICD, while others are known to remove immunosuppressive cell populations or reprogram aspects of the tumor microenvironment. As a result of these properties and others, adding PD-1/PD-L1 blockade to chemotherapy has already achieved some clinical success, with the most notable example to date being FDA approval of pembrolizumab, pemetrexed and carboplatin as first-line treatment for certain types of NSCLC in May 2017.
If trials that combine chemotherapy and RT are included, our search identified 216 ongoing trials, nearly half of which (104) are using pembrolizumab for PD-1/PD-L1 blockade. Excluding the 41 chemoradiotherapy trials, which were discussed in the previous section, yields 175 trials. We subdivided these trials by clinical trial phase and tumor site, finding 35 phase 3 and 68 phase 2 trials spanning dozens of tumor sites (Table 3). NSCLC is the most common site, with 36 trials ongoing, including 12 phase 3 trials. Breast cancer is the next most common site, with 26 trials in total and 5 phase 3 trials ongoing, followed by hematologic and ovarian cancers (Table 3).
Table 3.
Ongoing clinical trials combining chemotherapy with PD-1/PD-L1 blockade.
Some trials are enrolling multiple tumor types and were listed twice. Abbreviations: NSCLC = non-small cell lung cancer, SCLC = small cell lung cancer.
Tumor-targeted therapy
In this section we included agents with a multitude of tumor-targeted mechanisms. Like chemotherapies, these tumor-targeted therapies possess numerous immunomodulatory effects. Although somewhat difficult to generalize given the diversity of mechanisms of action, these agents not only promote direct tumor cell killing and subsequent release of immunostimulatory tumor-associated antigens but also can directly impact immune cells, which may also rely on the pathways targeted by these agents for critical functions. For example, MEK inhibitors are approved for melanoma patients with the BRAF-V600 mutation. As T cell activation also relies on this pathway, MEK inhibitors have direct effects on T cells as well.18
We found 261 trials combining tumor-targeted therapies with PD-1/PD-L1 blockade, 19 of which are phase 3 trials. Grouping these trials by mechanism of action revealed that the most commonly targeted pathways are VEGF (50, 37 of which are bevacizumab), B-Raf/MEK (22), EGFR (19) and BTK (17). For agents not directed at these highly represented targets, we were able to categorize by mechanism more broadly. We identified 49 tyrosine kinase inhibitors not directed against the targets above, 32 drugs with predominantly intranuclear targets like CDKs and PARP, 21 mAbs that function by antibody-dependent cell-mediated cytotoxicity (like rituximab), 22 thalidomide derivatives and 12 hormonal therapies (Fig. 3A). Several other targets/subgroups also have ongoing combination trials, and these are listed in Table 4.
Figure 3.
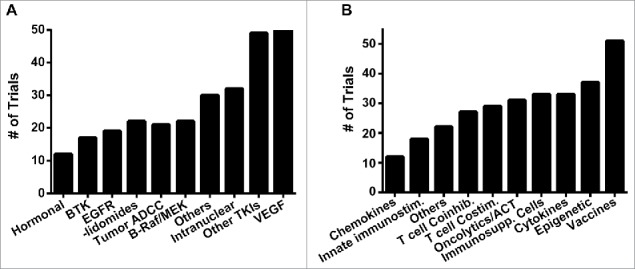
Analysis of trials combining tumor-targeted agents or immunotherapies with PD-1/PD-L1 blockade. Number of trials listed according to mechanism of action for A, tumor-targeted therapies, and B, non-CTLA-4 immunotherapies.
Table 4.
Ongoing clinical trials combining tumor-targeted therapies with PD-1/PD-L1 blockade.
Some trials were listed twice because they are assessing more than 1 targeted therapy. ADCC = antibody-dependent cellular cytotoxicity, ALK = anaplastic lymphoma kinase, BTK = Bruton's tyrosine kinase, CDK = cyclin dependent kinase, EGFR = epidermal growth factor receptor, HER2 = human epidermal growth factor receptor 2, mAb = monoclonal antibody, PARP = poly (ADP-ribose) polymerase, PI3 K = phosphoinositide 3-kinase, TKI = tyrosine kinase inhibitor, VEGF = vascular endothelial growth factor.
Immunotherapy
PD-1/PD-L1 blockade prevents negative regulation of T cells during the effector phase of antitumor immunity. Agents that can stimulate a more robust effector T cell response, such as mAbs against CTLA-4, should therefore augment the activity of PD-1/PD-L1 blockade.19 In fact, impressive clinical data using this combination of checkpoint inhibitors was first published in 2013, with subsequent studies confirming this initial analysis. It should not be surprising then that 183 trials were identified with combination CTLA-4 and PD-1/PD-L1 blockade. This total includes 119 using ipilimumab and 64 with tremelimumab, as well as 30 phase 3 studies (Table 5). Interestingly, this combination is frequently used as a framework upon which to build additional therapies, as 53 of the 183 studies add at least one additional therapy to combined CTLA-4 and PD-1/PD-L1 blockade.
Table 5.
Ongoing clinical trials combining CTLA-4 with PD-1/PD-L1 blockade.
Some trials are enrolling multiple tumor types and were listed twice. Abbreviations: AST = advanced solid tumors, CTLA-4 = cytotoxic T-lymphocyte associated protein-4, NSCLC = non-small cell lung cancer, RCC = renal cell carcinoma.
Beyond CTLA-4 blockade, we divided the remaining 285 immunotherapy studies into 10 subgroups based upon mechanism (Fig. 3B), and we further subdivided these groups by target, when applicable (Table 6). We identified 51 studies using vaccination, with the majority being tumor- or peptide-based vaccines (Table 6). The next most common class, with 37 trials found, is epigenetic agents, namely HDAC inhibitors like entinostat and the hypomethylating agent azacitidine. We found 33 trials each ongoing with cytokines, like IL-2 and interferons, and with inhibitors of immunosuppressive elements, like the indoleamine 2,3-dioxygenase inhibitor epacadostat and cyclophosphamide, which is known to deplete regulatory T cells.23 Directly cytotoxic immune-based agents like adoptive T cell therapy and oncolytic viruses have 31 ongoing trials. Therapies targeting T cell costimulatory receptors, such as CD40, 4–1BB or OX40, or coinhibitory receptors like LAG3, were the next most common with 29 and 27 trials, respectively. Next most common, with 18 trials, is the innate immunostimulatory group, which includes natural killer cell-targeted agents and TLR agonists. Finally, 12 trials combine PD-1/PD-L1 blockade with chemokines (Table 6). Of note, only 4 phase 3 trials were identified, 2 of which use azacitidine (NCT02951156, NCT03092674), 1 with epacadostat (NCT02752074) and 1 with T-VEC (NCT02263508).
Table 6.
Ongoing clinical trials combining IT therapies with PD-1/PD-L1 blockade.
Some trials are treating with more than one agent of an IT type and were listed twice. Abbreviations: A2 a = adenosine A2 a receptor, BCG = Bacillus Calmette-Guerin, BITE = Bispecific T cell engager, CCR = CC chemokine receptor, CSF = colony stimulating factor, CTX = cyclophosphamide, CXC = CXC motif, LAG3 = lymphocyte-activation gene 3, IDO = indoleamine 2,3-dioxygenase, IT = immunotherapy, mTOR = mammalian target of rapamycin, Tregs = regulatory T cells, Misc. = miscellaneous, HDAC = histone deacetylase, STAT3 = signal transducer and activator of transcription 3, TLR = toll-like receptor, STING = stimulator of interferon genes.
Discussion
In this review we identified 1,218 ongoing clinical trials with a planned enrollment of 227,190 patients. By comparison, a search of clinicaltrials.gov for open interventional trials with the term “cancer” in June 2017 retrieved 13,112 results. Thus a single signaling pathway accounts for approximately 10% of all ongoing clinical trials in oncology. Perhaps more striking is that over 75% of these 1,218 trials (916) combine PD-1/PD-L1 blockade with at least one additional therapy. These combination trials span the spectrum of cancer treatment modalities and tumor sites, and are being initiated with multiple PD-1/PD-L1 mAbs.
One of the most fascinating trends identified in this analysis involves CTLA-4 blockade. As noted above, over 50 trials were identified that essentially replace single agent PD-1 blockade with combined PD-1/CTLA-4 blockade as the foundation to which additional agents are added. In the coming years, this scenario will likely repeat itself as additional immunotherapeutic agents show additive or synergistic benefit with PD-1/PD-L1 monotherapy with more favorable toxicity profiles.
While this example highlights the potential clinical benefit that combination immunotherapies hold, it also suggests that biologic plausibility will not be the limiting factor to cap the currently unsustainable rate of clinical trial initiation. Fig. 1 A shows that the growth of PD-1 clinical trials has been and continues to be exponential, with the number of trials essentially doubling every year. Thus, over 10,000 trials would be ongoing in 2020 if that rate were to continue, an untenable amount due to funding and patient enrollment limitations rather than potential trials.
This issue is one of many that the field currently faces. Several other challenges, including clinical trial design, development of preclinical models, enhanced toxicity, and clinical trial endpoints, have recently been summarized by the Society for Immunotherapy of Cancer Task Force.24 Overcoming these challenges will take a focused effort to allocate a finite pool of resources to the most promising combinations.
The broad clinical activity, relatively low side effect profile and appealing mechanism of action has elevated PD-1/PD-L1 pathway blockade to the forefront of oncologic clinical study. With nearly 1,000 combinatorial clinical trials ongoing and the rate of trial initiation increasing, these agents will continue to transform cancer treatment options for the foreseeable future.
Methods
Using a “first received” end date of April 30, 2017, we queried clinicaltrials.gov for “PD-1” and “PD-L1” and for these specific PD-1/PD-L1 blocking agents: pembrolizumab, nivolumab, atezolizumab, durvalumab, PDR001, avelumab, pidilizumab, REGN2810, SHR-1210, MEDI0680, JS001, LY3300054, JNJ-63723283, FAZ053, BGB-1317, AMP-224, KN035, JNJ-61610588, BCD-100, MGA012 and SHR-1316. Only those trials administering PD-1/PD-L1 mAbs to cancer patients and with a status of “Not yet recruiting,” “Recruiting,” or “Active, not recruiting” were included. GraphPad Prism was used for statistical analysis.
No conflicts of interest
There was no direct funding for this work.
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. PMID:22658128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. PMID:25858804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. PMID:22658127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brawley L. With 20 Agents, 803 Trials, and 166,736 Patient Slots, Is Pharma Investing Too Heavily in PD-1 Drug Development? Cancer Lett. 2016:Vol 42. [Google Scholar]
- 5.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. doi: 10.1186/s12929-017-0329-9. PMID:28376884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton DL. Changing concepts of cancer surgery: surgery as immunotherapy. Am J Surg. 1978;135(3):367–371. doi: 10.1016/0002-9610(78)90067-3. PMID:343622. [DOI] [PubMed] [Google Scholar]
- 7.Mickisch GH, Mattes RH. Combination of surgery and immunotherapy in metastatic renal cell carcinoma. World J Urol. 2005;23(3):191–195. doi: 10.1007/s00345-004-0468-y. PMID:15791469. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AM, Chiarion-Sileni V, Grob JJ, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. PMID:25840693. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CB, Jagsi R. The Promise of the Abscopal Effect and the Future of Trials Combining Immunotherapy and Radiation Therapy. Int j Radiat Oncol, Biol, Phy. 2016;95(4):1254–1256. doi: 10.1016/j.ijrobp.2016.02.067. PMID:27354132. [DOI] [PubMed] [Google Scholar]
- 10.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. PMID:22397654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. doi: 10.1016/j.ctrv.2015.03.011. PMID:25872878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. PMID:23291374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33(51):7415–7422. doi: 10.1016/j.vaccine.2015.05.105. PMID:26148880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacchelli E, Bloy N, Aranda F, Buqué A, Cremer I, Demaria S, Eggermont A, Formenti SC, Fridman WH, Fucikova J, et al.. Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology. 2016;5(9):e1214790. doi: 10.1080/2162402X.2016.1214790. PMID:27757313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett. 2015;368(2):185–190. doi: 10.1016/j.canlet.2015.03.024. PMID:25799953. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. PMID:26678337. [DOI] [PubMed] [Google Scholar]
- 17.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al.. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. PMID:27745820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, et al.. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. doi: 10.1038/nrc.2017.17. PMID:28338065 [DOI] [PubMed] [Google Scholar]
- 19.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. PMID:22658126. [DOI] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al.. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. PMID:26027431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. PMID:23724867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff F, Leisch M, Greil R, Risch A, Pleyer L. The double-edged sword of (re)expression of genes by hypomethylating agents: from viral mimicry to exploitation as priming agents for targeted immune checkpoint modulation. Cell Commun Signal. 2017;15(1):13. doi: 10.1186/s12964-017-0168-z. PMID:28359286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DT Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. PMID:22761338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. JITC. 2017;5:16 PMID:28239469. [DOI] [PMC free article] [PubMed] [Google Scholar]