Fig. 6.
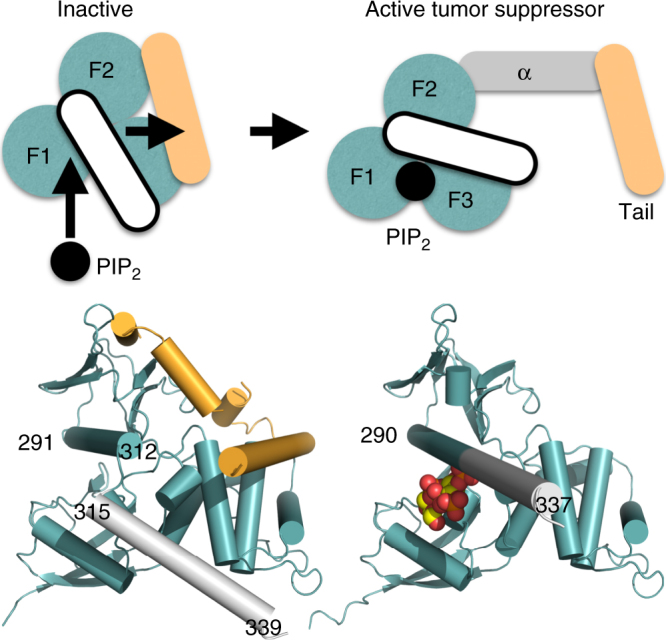
Activation of the neurofibromin 2 tumor-suppressive function. In its inactive state, neurofibromin 2 is in a closed conformation through interactions of the FERM domain (teal; F1, residues 18–98; F2, residues 111–213; F3, residues 220–312) and the tail domain (pale orange). The α-helix C-terminal of F3 (residues 315–339; white) does not interact with the tail domain. PIP2 binds to F1 and the last α-helix (residues 291–312; not depicted) of F3, thereby causing the last F3 α-helix (residues 291–312) and the following α-helix αH (residues 315–339) to rearrange as one long and continuous α-helix (residues 290–337), thereby displacing the tail domain and severing the head–tail interaction which results in active tumor suppressor functions. The central α-helical domain is shown in gray. The head/tail neurofibromin 2 crystal structure (head structure from PDB entry 1isn34; tail structure from PDB entry 4zrj35) is shown below the schematic on the bottom left and our PIP2-bound structure on the bottom right
