Figure 1.
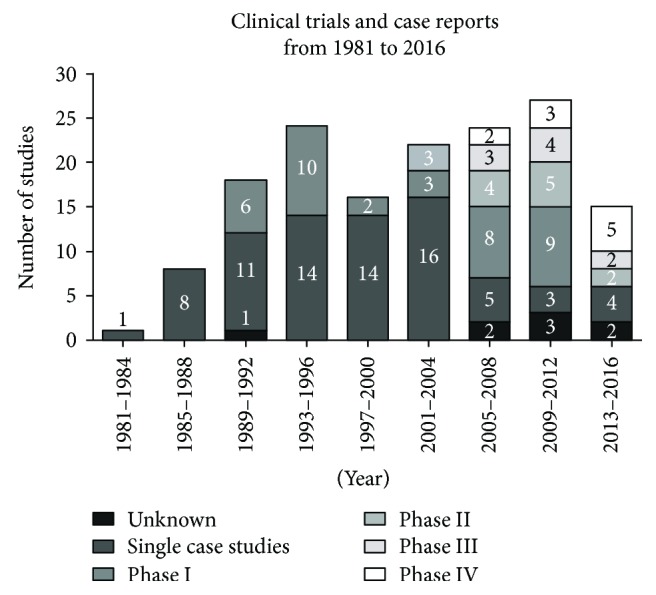
Number and type of clinical trials for stem cell-based skin regeneration since 1980. Single-case studies or small series of single cases were the main clinical activities reported before 1989. Subsequently, the number of phase I trials (safety) increased, reaching its first peak in the mid-1990s, followed by phase II studies (safety and secondary efficacy endpoints) since the early 2000s. Large, late-stage clinical phase III (efficacy) and phase IV (surveillance of products on market) studies were reported since 2005, which was accompanied by a drop in single-case reports. Remarkably, no additional phase I clinical trial has been launched since 2013. Only studies reported in http://pubmed.com and/or listed on http://clinicaltrials.gov were included in this analysis. The search was restricted to studies reported between 1981 and 2016.
