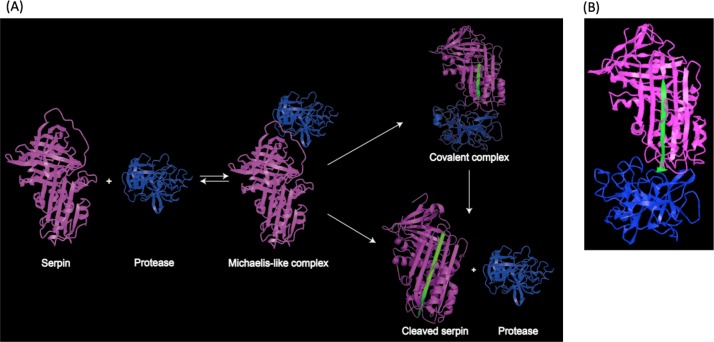
Figure 2. Representation of serpin-protease interaction.
(A) Proposed process of serpin-protease interaction. A serpin (magenta) interacts with targeted protease (blue), and the Michaelis-like complex of serpin and protease is formed. The complex either undergo peptide bond hydrolysis resulting in a kinetically trapped loop-inserted covalent complex (inhibitory pathway), or a cleaved serpin and free protease (non-inhibitory/substrate pathway). The cleaved and inserted RCL is highlighted in green. Serpin-protease complex is stable. Possibility of transition from covalent complex to cleaved form exists yet slim, since complex in vivo would be cleared long before complex decay could occur. (B) Structure of stable serpin- protease complex (PDB: 2D26). The complex is formed by serpin α1PI (magenta) and protease elastase (blue). The inserted RCL is highlighted in green.